 The 3D Structure of GFP
The 3D Structure of GFP
 The 3D Structure of GFP
The 3D Structure of GFP
Yang et al. [6] have solved the crystal structure of recombinant wild-type A. victoria GFP to a resolution of 1.9 Å. The three-dimensional structure of the S65T variant of A. victoria GFP has been determined at 1.9 Å resolution by Ormö et al. [7].
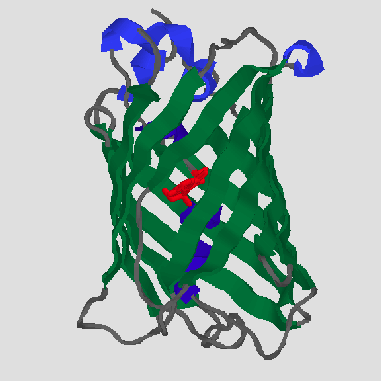
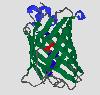
GFP is the representative of a new protein fold, which Yang et al. [6] have named a beta-can. On the outside, 11 antiparallel beta strands (green) form a very compact cylinder. Inside this beta-structure there is an alpha-helix (dark blue), in the middle of which is the chromophore (red). There are also short helical segments (light blue) on the end of the can. The cylinder has a diameter of about 30 Å and a length of about 40 Å
The very compact single-domain structure with the chromophore centrally located in the molecule and protected from bulk solvent can explain a number of characteristics of GFP, such as:
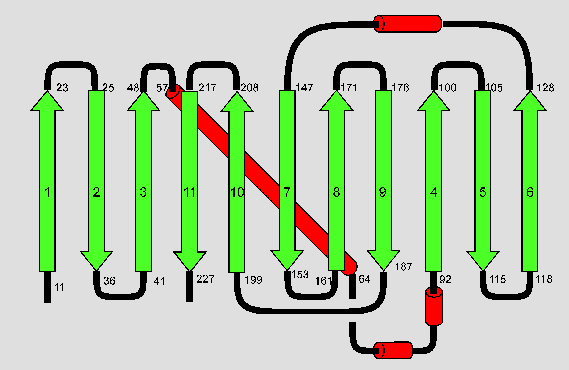
The above diagram illustrates the topology of the folding pattern. Beta-strands are shown in green, alpha-helices in red and connecting loops in black. The numbers of the residues at the beginning and end of the secondary structure elements are given as well.
Ormö et al. [7] have found basically the same topology for the S65T variant. They claimed an additional short alpha-helix at the N-terminus, a loop instead of a helix between beta-strands six and seven and they disagree about the exact beginnings and ends of secondary structure elements. However, all these differences can most probably be explained by the use of different algorithms for secondary structure determination.
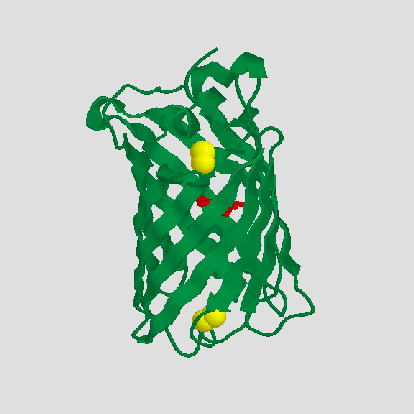
There are two cysteines (shown in yellow), Cys48 at the beta-strand 3 and Cys70 within the inner
helix, which do not form a disulfide bond. Cys48 may be partially solvent exposed,
whereas Cys70 is buried within the core of the protein.
This structural information sheds new light the work of Inouye and Tsuji
[10],
who found that GFP fluorescence is reversibly lost upon reduction with strong
reducing agents such as sodium dithionite, but not upon treatment with weaker
reducing agents known to reduce disulfide bonds, such as beta-mercaptoethanol
(BME), dithiothreitol (DTT) or reduced glutathione (GSSG). However, fluorescence
was irreversibly eliminated by treatment with the sulfhydryl reagent
dithiobisnitrobenzoic acid. The authors concluded that a free cysteine residue
may be required for fluorescence.
Considering the structure of GFP, it is
obvious why BME. DTT and GSSG do not have any effect, as there is no disulfide
bridge that might be reduced. It seems quite possible that the strong reducing
agents reduce the dehydro-Tyr of the fluorophore itself, thus destroying
the large delocalized pi-system. In view of the proposed mechanism of
fluorophore formation it is perfectly plausible that
such a reduction is reversible. Dithiobisnitrobenzoic acid covalently binds to
free SH-groups. Cys70 is indeed quite close to the fluorphore, the distance
between its gamma-sulfur and the oxygen of Gly67 being about 6.2Å, so it
might have an influence on fluorescence. However, it seems more likely that
the structure of GFP would be severly disrupted by a covalent modification of
Cys70 with such a bulky reagent. How modification of Cys48 could eliminate
fluorescence is not quite clear, but so far it has not been established which
of the two cysteines or whether both have reacted with dithiobisnitrobenzoic acid.
The fluorophor is located almost at the center of the cylinder and is completely
protected from bulk solvent [6,7], which makes
it quite impossible for an enzyme to access the fluorophore and catalyze its
formation and thus underlines the hypothesis of autocatalytic fluorphore
formation.
The plane of
the chromophore is roughly perpendicular to symmetry-axis of the cylinder
[7].
Ormö et al. [7] have found a large cavity
on one side of the chromophore, which does not open to bulk solvent and which
they assume to be filled with water. None of this has been mentioned by
Yang et al. [6].
George Phillips has mounted his whole paper on the structure of GFP, which includes a model of the fluorophore and its environment (Fig.4). The surprising number of charged and polar residues has been mentioned before, as they presumably influence the Förster cycle of the fluorophore. It has also been suggested that Arg96 may catalyze the backbone-cyclyzation step in fluorophore formation [7].
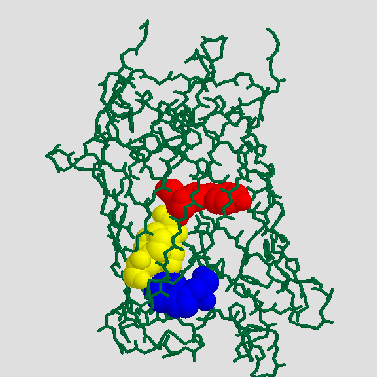
GFP has a single tryptophan, Trp 57 (blue), which is located 13 to 15 Å away from the chromophore (red) [7]. Trp 57 is seperated from the chromophore by Phe 64 and Phe 46 (yellow) [6] and the long axis of its ring system is nearly parallel to the long axis of the chromophore [7]. Therefore, an efficient energy transfer from Trp to the chromophore should be possible, which explains why no seperate tryptophan emission can be observed.
![]() [GFP Chromophore]
[GFP Chromophore]
![]() [Index]
[Index]
![]() [References]
[References]
 Silke Jonda's PPS2 project
Silke Jonda's PPS2 project