 Structure and Function of Green Fluorescent Protein
Structure and Function of Green Fluorescent Protein
About this project
These webpages about Green Fluorescent Protein (GFP) have been developped as
a dissertation project for the
Principles of Protein
Structure Using the Internet course, in which I have participated as a
student. You may also want to have a look at my colleagues'
projects.
At this point I would like to thank all the tutors, consultants and fellow
students,
who made this course work - and it has often been great fun, too!
I am also indebted to Prof.
George Phillips, who has kindly given me the coordinate file of GFP prior
to its PDB release. Finally, I am grateful to Horst-Joachim Schirra for his
help with corel-draw and for patiently discussing many ideas of this project
despite his own heavy workload.
The project is best viewed with Netscape2, but apart from a few messy sub- and
superscripts Netscape1 should be okay as well.
Feel free to mail me
your comments!
Abstract
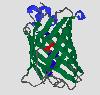
GFP is an extraordinary protein in many respects: It is fluorescent and its
fluorophore is made up of modified amino acid residues. Moreover it is the first known
example of a Förster cycle within the core of a protein. Furthermore its
crystal structure has recently been solved and the protein turned out to have
a new structural motif, called the beta-can. On the following pages I will set
out to discuss some of the most interesting features of this unique protein,
starting out with its function in the introduction, followed by a view on the
more or less isolated chromophore and finally presenting the three-dimensional
structure of this fascinating protein.
Content
Introduction
- The in vivo role of GFP
- Some basic properties of A. victoria GFP
The Chromophore of GFP
- Chemical Structure of the Chromophore
- Biosynthesis of the Chromophore
- Excitation and Fluorescence Emission Spectra of GFP
- Förster cycle
- Mutations affecting the of Tyr66
- Mutations of the chromophore forming residues
The 3D Structure of GFP
- The Beta-Can Structure
- Topology of Folding
- Cysteins
- The Environment of the Fluorophor
- Tryptophan Fluorescence
References
 [Introduction]
[Introduction]
 Silke Jonda's PPS2 project
Silke Jonda's PPS2 project
Structure and Function of GFP
updated 28.11.96
 Structure and Function of Green Fluorescent Protein
Structure and Function of Green Fluorescent Protein
 Structure and Function of Green Fluorescent Protein
Structure and Function of Green Fluorescent Protein
 Silke Jonda's PPS2 project
Silke Jonda's PPS2 project