A large number of bacterial responses involve the interaction of two regulatory proteins in a two-component signal transduction system. These two-component systems are ubiquitous in bacteria and in their simplest form consist of a sensor that monitors some environmental parameter, usually located in the cytoplasmic membrane and a cytoplasmic response regulator that mediates an adaptive response, usually a change in gene expression.
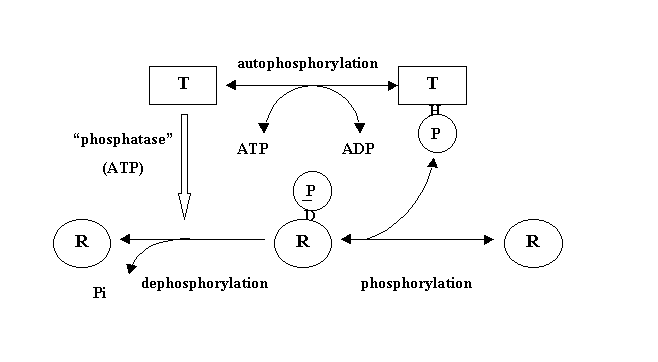
The sensor component is a histidine protein kinase. The N-terminal domain of this protein kinase functions as the input region, detecting environmental stimuli directly or interacting with an upstream receptor. Many sensor domains are embedded in the cellular membrane. Extremely varied sensor domain - reflects variety of signals. Kinases are activated by a signal to phosphorylate the response regulator. In the absence of a stimulatory signal most are phosphatases of their phosphorylated response regulators. Fine-tuning of the transcription level is directed by a response regulator to correspond to the level of signal input.
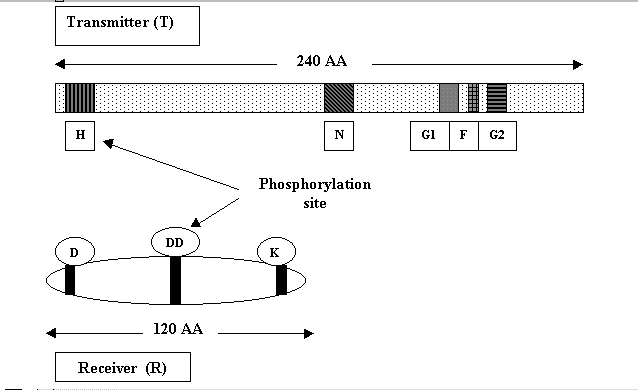
Sequence analysis shows that C-terminal domain of this family of proteins is highly conserved and is approximately 240 amino acids long with several blocks of nearly invariant residues. One of these, termed the H box, includes a histidine residue at which the protein autophosphorylates using the gamma-phosphoryl group of ATP. The G1 and G2 boxes are glycine-rich sequences that resemble the nucleotide-binding motifs of other proteins. The specific functions of the F and N boxes are not apparent from inspection of their sequences but presumably are involved in the catalytic activity of the protein.
In addition to their kinase activities, histidine protein kinases such as EnvZ and NtrB also act as phosphoprotein phosphatases, accelerating the dephosphorylation of their cognate response regulators, OmpR or NtrC, in an ATP-dependent fashion. The combined kinase and phosphatase activities of the protein kinases thus act to control the amount of the phosphorylated form of the response regulator present in the cell, in response to environmental signals.
The protein kinases can be divided into two groups: membrane-associated proteins such as EnvZ or PhoR of E.coli and cytoplasmic proteins such as CheA of E.coli and NtrB of E.coli and Klebsiella pneumoniae. The N-terminal transmembrane and periplasmic domains of EnvZ protein kinases are directed towards sensing environmental signals. Cytoplasmic protein kinases are thought to respond to intracellular signals. Many of the protein kinases identified in Gram positive bacteria have membrane-spanning segments in their N-terminal domains.