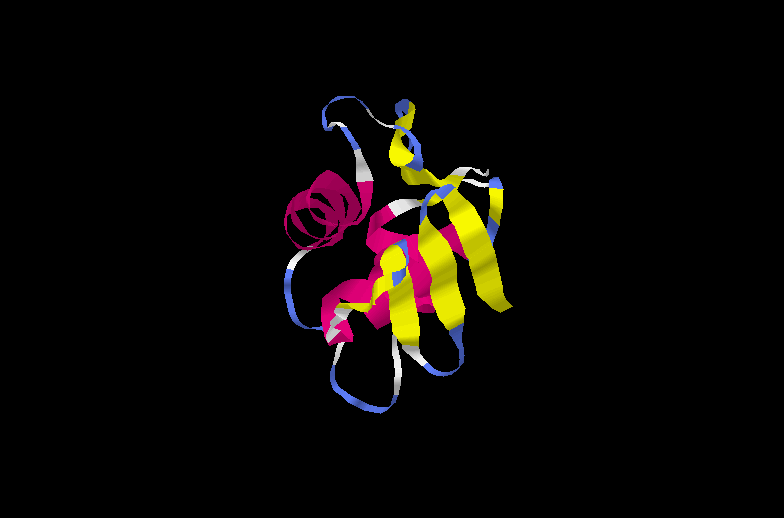
Phosphorylated OmpR binds to the promoters of the porin genes to regulate their expression. OmpR is a two-domain structure, an N-terminal phosphorylation domain joined to a C-terminal DNA binding domain by a flexible linker region. OmpR is a member of a subfamily of at least fifty response regulators with homologous C-terminal DNA-binding domains of approximately 98 amino acids.
The C-terminal domain of OmpR consists of three alpha helices packed against two antiparallel beta sheets. Alpha helices 2 and 3 and the 10 residue loop connecting them constitutes a helix-turn-helix motif. Alpha helix 3 and the connecting loop between the two C-terminal beta strands 6 and 7 are probably the DNA recognition sites. A large loop between alpha helices 2 and 3 is the site of interaction with the RNA polymerase alpha subunit. OmpR is phosphorylated on an aspartyl residue as described previously. This induces a conformational change that is transmitted to the C-terminal domain by means of the flexible linker.
Phosphorylation of the highly conserved family of effector proteins is considered to occur at one or more aspartate residues (Asp-11, Asp-12 and/or Asp-55. The creation of mutants with aspartate to alanine substitutions at positions 11, 12 and 55 demonstrates that Asp-55 is the primary phosphate acceptor site in OmpR, Asp-11 may also serve as a phosphorylation site.
OmpR acts as a positive as well as a negative regulator of ompF expression by binding to DNA sequences in the ompF promoter region. The DNA binding activity of OmpR is itself regulated by phosphorylation through EnvZ. Phosphorylation is thought to change OmpR from an activator to a repressor molecule. Phosphorylation causes binding of OmpR to a DNA region between the -39 to -100 region of the OmpF promoter. As the amount of OmpR phosphate increases, a binding site located at a further upstream -361 to -380 region is occupied which has been reported as important for OmpF repression. Six OmpR molecules bind to the -100 to -39 sequence of the ompF promoter which consists of three 20 base units F1, F2 and F3, each of which is bound by two OmpR molecules that bind in tandem to two direct repeats in each of the F units. Two further OmpR molecules bind to the -380 to -361 distal site. Cooperative interactions play a role in enhancing binding of OmpR to lower affinity F2 and F3 sites. Evidence suggests that the RNA polymerase alpha subunit interacts with certain transcriptional activators to inhibit expression of the porin genes.
Summary of OmpR structural properties in CATH database