The Glycogenolysis Cascade
Glucagon binds to specific receptors in the plasma
membrane of liver cells, called 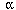 1-adrenergic receptors
causing an allosteric change in the receptor.
1-adrenergic receptors
causing an allosteric change in the receptor.
On the cytoplasmic face of these receptors are heterotrimeric
G-coupled proteins
composed of the alpha, beta and gamma subunits. Upon
glucagon binding, the alpha subunit releases the bound GDP in
exchange for GTP.
The GTP-bound
alpha subunit is released of its inhibitory
beta/gamma subunits and binds to adenylate cyclase.
The binding of the GTP-bound alpha subunit to adenylate
cyclase triggers the production of
cyclic AMP
(cAMP) from ATP. cAMP is a small molecule
second messenger which acts on the cAMP dependent protein kinase,
cAPK, also known as protein kinase A, PKA.
cAPK
is an oligomeric protein consisting of two regulatory
and two catalytic subunits. In the absence of cAMP,
this tetrameric complex is inactive. When cAMP is present,
the binding of two molecules of cAMP to each of the regulatory
subunits leads to the dissociation
of the tetrameric complex. The dissociated catalytic subunits are now
enzymatically active
and capable of phosphorylating
serine or threonine residues of target proteins.
Glucagon signals a need for an
increase in blood glucose level. Therefore, the activated cAPK
has a dual
function. By
phosphorylating glycogen synthase, cAPK inhibits the synthesis
of glycogen. cAPK also phosphorylates glycogen phosphorylase kinase,
the penultimate enzyme in the pathway of glycogen degradation.
Glycogen phosphorylase kinase then phosphorylates glycogen
phosphorylase converting it into the active form, glycogen
phosphorylase a, gpa capable of
degrading glycogen to glucose 1-phosphate.
BACK
NEXT
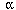 1-adrenergic receptors
causing an allosteric change in the receptor.
1-adrenergic receptors
causing an allosteric change in the receptor.