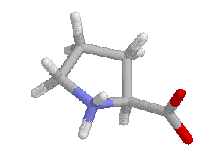

Proline has restrictions in phi-psi space that arise from the 5-membered ring. Phi is restricted to approxamatly -60 by the ring and psi angles fall into two groupings near -45 and +135 in the helical and sheet regions of the Ramachandran plot. The beta carbon and amino hydrogens of the residue proceeding proline is sterically restricted by the delta carbon bound to the imide nitrogen. An example Ramachandran plot from Procheck is shown below. It has the values of over 160 high resolution structures as the shaded region with values for a typical protein as data points.
Prolines are frequently found in the first, N-terminus turn of a helix where the loss of the H-bond to the immino nitrogen does not cause significant effects. In an analysis of high resolution structures, proline most frequently occured as the second residue of a helix, with almost 90% of prolines in helices occuring in the first turn (1,3). Prolines in alpha helices after the first turn (4th residue) cause a kink in the helix.This kink is caused by proline being unable to complete the H-bonding chain of the helix and steric or rotamer effects that keep proline from adapting the prefered helical geometry. The kink is `away' from the proline residue,which is frequently on the hydrophilic side of the helix.
Poly-l-proline has been found to occur in two unique helical conformations, I and II.
Type I is a right handed helix with phi= -83 and psi = 158 and 3.3 residues per turn. All of the residues in the type I helix are cis prolines. Type II is an all trans, left handed helix with phi= -78 psi= 149 and 3 residues per turn. This conformation is found in proline containing peptides bound to SH3 domains.
Proline is not favored in beta sheet structures as it cannot complete the H-bonding network. When proline does occur in sheets, it may be in a bulge or sheet edge where the lack of an amino hydrogen bond doner is not critical.
Type phi(i+1) psi(i+1) phi(i+2) psi(i+2)
I' 60 30 90 0
II -60 120 80 0
II' 60 -120 -80 0
VIa -60 120 90 0
VIb -120 120 -60 150
Proline can occupy the i+1 position in type I and II turns. The phi angle for these types of turns is -60, near that of trans proline.
Type VI, or cis-proline loops have proline in a cis conformation. In type VIa, the turn is stabilized by hydrogen bonding between the first and fourth residues of the turn. In type VIb, that has no stabilizing hydrogen bonding. Both can be stabilized by aromatic stacking if the residue proceeding proline is phenylalanine or tyrosine.
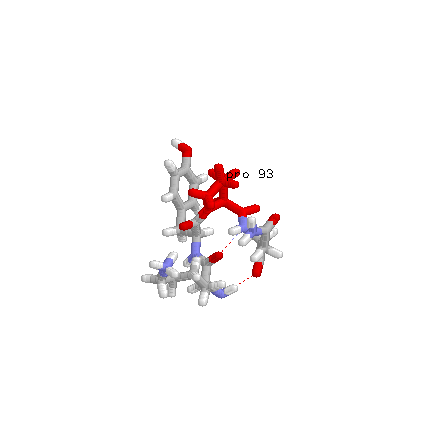
Residues 57-60 from PDB entry 2SN3