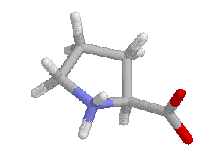

Collagen is the major structural protein found in tendons. ligaments, skin and bones. It has the repetitve sequence Gly-X-Y where X and Y are frequently proline. It forms a three-stranded triple helix with each helix in a conformation similar to left-handed poly-proline II. The prolines provide elasticity and strength due to the limited conformations proline can assume and the glycines are too close to the other strands of the helix to allow room for a side chain. The helix is held together by hydrogen bonds from the glycine NH to the carbonyl of residue Xaa of another strand. This also allows proline to occur in collagen as the amino H of residues Xaa and Yaa is not strictly required for collagen as it is for other regular structures such as helices and sheets. Many of the prolines are hydroxylated at the 3 and 4 positions of the proline ring by prolyl hydroxylases that require vitamin C. This modification occurs before the collagen is folded into a three-stranded helix and the hydroxals stabilizes the helix by interchain hydrogen bonds.
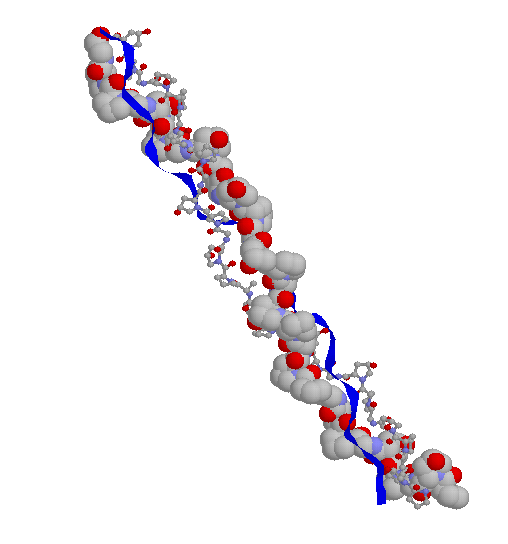
Strands shown as ribbon, spacefill and wireframe.
PDB entry 1CAG rendered with RASMOL