|
Let's now look at one more molecule. It has the following SMILES string: |
|
COC(=O)C1C2CCC(CC1OC(=O)c3ccccc3)N2C |
| Can you draw it? And if you can, do you know what it is?
|
| |
|
If you cannot draw it, all is not lost. Although most services that will produce
a 3D view of your molecule are proprietary, there are some services on the web
that will at least produce a 2D diagram from a SMILES string. Try for example
the following: |
|
Xemistry web sketcher. |
|
Paste in the SMILES string from above and press
Enter.
This is a 2d diagram and it would be nice to have a 3D structure to look at. I used
to access the Chemistry Development Kit showcase to do this, but it has temporarily disappeared from the web.
As an alternative,
there is an online demo of CORINA, a highly regarded 3D generator from Gasteiger's lab (now
marketed by Molecular Networks GmbH). Go to:
http://www.molecular-networks.com/online_demos/corina_demo.html)
and enter the SMILES string from the previous paragraph, and then press
Submit . This should generate
a 3D structure for you which you can rotate and take a better look at (you need to have Java 1.4 or higher
and JavaScript enabled in your browser for this to work). If you cannot see
it, you can still most likely download the structure as PDB or MOL and
open it in Chimera instead.
Having seen the molecule in 3D, do you recognise it now?
|
| |
|
If not, try to see if you can find it in another database. We will try ,
one of the largest
collections of small molecule structures available on the web. This service used to be totally open, but they
are now asking users to register. We will instead login as guests for this tutorial. Go to
the URL: |
|
http://chembank.broadinstitute.org/ and select Enter as guest. |
| Under Find small molecules select
by similarity and enter the SMILES string for your molecule
in the text box for SMILES. Set the threshold to 0.95.
The search is very fast and when I did it it returned 6 molecules.
If ChemBank fails try PubChem instead (ChemBank seems to have issues this year).
The molecule you are looking for has ChemBankID: 1555. Note that the entry with ChemBankID 3560825
has the same molecular weight and appears to be the same molecule, except that the stereochemistry
is defined
(think whether this is a good idea - having molecules with or without
stereochemistry with different ids and no obvious links from one to the other...). Check whether the two compounds
are indeed identical molecules (HINT: Remember the property of InChIs?). So your mystery
molecule was none other than .
Note that if you sort the results by descending Tanimoto score, the fourth hit
(at least on my search it was fourth, ChemBankID 1548, benzoylecgonine, similarity 0.99)
is the major metabolite of cocaine in the body (i.e. the molecule that cocaine is metabolised into
by the body's cholinesterase proteins), it is water-soluble and it is excreted in the urine, enabling
the detection of cocaine use by urine drug tests.
|
| |
|
Cocaine works by affecting neurotransmission in the brain and the rest of the body.
Interactions between nerve cells in the brain are responsible for our mood, thoughts
and motivation for action. Nerves communicate through the release of chemicals
known as neurotransmitters. One nerve releases a chemical which
stimulates or inhibits
a second nerve. The chemical however is then reabsorbed by the first nerve to
avoid overstimulation. In the brain, the release of dopamine in the so-called
"reward pathway" is what makes us feel good during activities that support the survival
of our species (eating, having sex, drinking water etc). Cocaine acts by blocking
the reuptake of dopamine, and thus enhancing the stimulation of the nerve cells and
making the user feel euphoric and powerful. Of course, there is a catch. Eventually
nerve cells become insensitive to increased dopamine levels (so you need bigger doses
to achieve the same effect), and the nerves releasing dopamine need time to build up
more of this chemical, since they can't simply reabsorb it. In the rest of the
body cocaine blocks the re-uptake of the neurotransmitter noradrenaline. This is
the cause of many of the side effects of its use, such as heart irregularities and
increased blood pressure.
|
| |
|
One might expect cocaine to strongly resemble the neurotransmitters whose re-uptake
it blocks, but similarity at the 2D level is relatively limited.
Can you find what
dopamine and noradrenaline look like? (try, for example,
KEGG). Does dopamine look
like noradrenaline? Can you find a reaction in KEGG that relates these
two compounds? |
| |
|
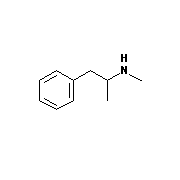
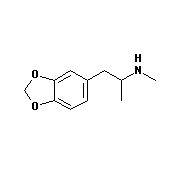
There are many drugs that interfere with neurotransmission, and some of them
do have a strong resemblance to dopamine and noradrenaline. Examples (see
diagrams below) include
amphetamine, methamphetamine (speed), and 3,4-methylenedioxymethamphetamine (ecstasy).
|
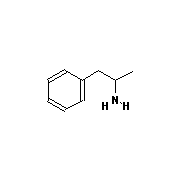 |
 |
 |
| Amphetamine |
Methamphetamine |
3,4-methylenedioxymethamphetamine |
|
|
If you want to have a look at drug-like compounds resembling these, try
DrugBank. Go to the following URL:
http://www.drugbank.ca/ |
|
Follow the link to Search and then
ChemQuery Structure Search
In the Marvin sketch box press Import
and select Paste Source SMILES and copy and paste in the
following: CC(N)Cc1ccccc1.
This is the SMILES string for the amphetamine molecule. Select a
Similarity Threshold, say 0.9. You will get back a series of molecules
that are either approved drugs, failed drugs or drugs under investigation.
|
| |
| This is the end of the first practical for the Chemoinformatics part of the
Structural Bioinformatics module. Congratulations!
|
| |

|