

|
|
|
|
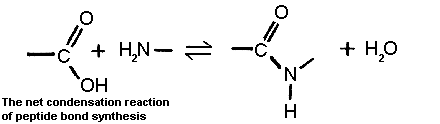
In this section the main properties of the 20 different amino acids found in genetically encoded proteins will be outlined. All amino acids have amino and carboxyl groups attached to the alpha-carbon atom. When an amino acid is incorporated into a polypeptide by the ribosome at say position i in the sequence, it undergoes a condensation reaction in which the carboxyl group of the preceding amino acid (i-1) forms an amide (or peptide) bond with the amino group residue i. In the next elongation cycle of the ribosome, the carboxyl group of residue i becomes covalently linked to the amino group of residue i+1 in the final sequence by another peptide bond. Hence all amino acids in the protein are linked by peptide bonds. In each condensation reaction an -OH group is essentially lost from the participating carboxyl group and a hydrogen is lost from the amino group, ie water is eliminated. The amino group becomes a trigonal >N-H during this process.
The three-dimensional structure and function of a protein are dependent on the sequence of amino acid side chains in the polypeptide. Amino acid side chains can be divided into several different classes based on their physico-chemical properties. The below diagram indicates the colour scheme for the atoms in this section. Hydrogen atoms are not shown for clarity and because they cannot be located in most protein X-ray structures. In the diagrams the main chain carboxyl group of each amino acid is shown only as a carbonyl (ie C=O) group as found in polypeptides.
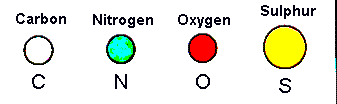
Last updated 9th Oct'96