

|
|
Domains in Proteins
Even as the first structures were solved, proteins were found to have
structurally distinct lobes (Phillips, 1966). However, the term "domain"
was not assigned to compactly folded structures until later when Wetlaufer
(1973) recognised it to be a common feature in unrelated proteins. Since
then, it has become clear that domains form an important level in the
hierarchical organisation of the three-dimensional structure of globular
proteins, although not all proteins can be described as multidomain structures.
Domains of recently evolved proteins are frequently encoded by exons, reflecting gene fusion of simpler modules. For example, in the case of hepatocyte growth factors and plasminogens, a number of kringle domains are present.
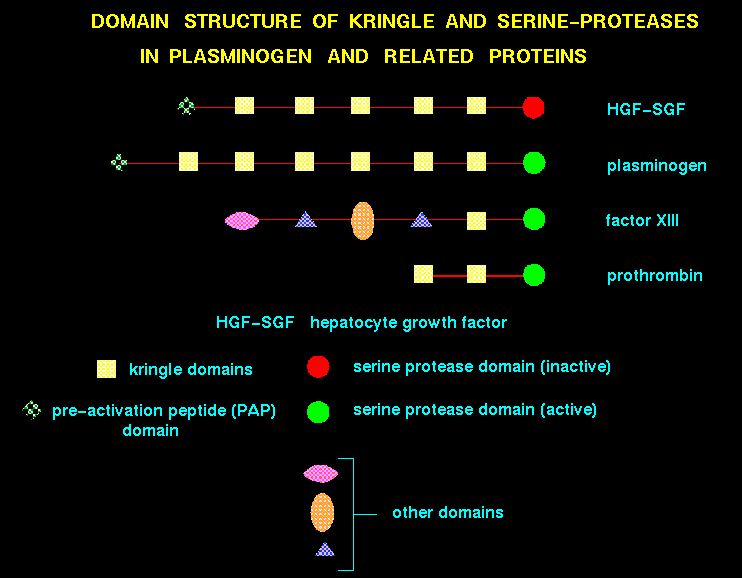
For a recent review on domain insertion, see Russell (1994). Domain swapping between two protomers is not uncommon (for example in the case of diphtheria toxin (Bennet et al., 1994)).
Several algorithms for domain identification have been suggested.
-by Stephen D. Rufino, Dept. of Crystallography, Birkbeck College
Domains are important both in protein folding and in biological function. They are found in different combinations and often bring with them discrete functions. The relative disposition of domains in a protein may be important for the function and/or ligand binding. Domain motions are important for a variety of functions.
There are other useful references in this area.

|
|
Last updated 7th April '97