

|
|
All-Beta Topologies
Again, this is a review of some of the 'classic' examples.
You should also browse through the Mainly Beta Class of the CATH Protein Structure Classification Database at University College, London. This lists 7 different architectures.
You should also be aware of alternative classifications, such as the Structural Classification of Proteins database (Alexey G. Murzin, Steven E. Brenner, Tim J.P. Hubbard, and Cyrus Chothia). In all, fifty-two categories of all beta folds are listed. A number of the entries have links to diagrams by Manuel Peitsch.
With MAGE installed, study this Kinemage on Alpha Domain Structures, which accompanies the Branden and Tooze book.
The immunoglobulin fold is introduced in the section on mosaic proteins . In this fold, the strands form two sheets packed against each other, forming a "beta sandwich". Also look at the fibronectin type 3 domain in the same section.
The packing arrangements in both aligned and orthogonal beta-sandwiches has been described in Section 9.
 Click here for a diagram
of this beta sheet arrangement in the lipocalin intestinal fatty acid-binding
protein.
Click here for a diagram
of this beta sheet arrangement in the lipocalin intestinal fatty acid-binding
protein.
![]() 1ifb (95Kb)
[Bbk|BNL|ExP|Waw|Hal]
... SCRIPT
1ifb (95Kb)
[Bbk|BNL|ExP|Waw|Hal]
... SCRIPT
This is just one member of the Lipocalin family, which bind small molecules between the sheets of the sandwich.
Some antiparallel beta sheet domains are better described as beta barrels rather than beta sandwiches, for example streptavadin.
![]() 1stp (87Kb)
[Bbk|BNL|ExP|Waw|Hal]
See also the diagrams of porin in the section on
membrane proteins in the previous chapter.
Note that some structures are intermediate between the extreme barrel and
sandwich arrangements.
1stp (87Kb)
[Bbk|BNL|ExP|Waw|Hal]
See also the diagrams of porin in the section on
membrane proteins in the previous chapter.
Note that some structures are intermediate between the extreme barrel and
sandwich arrangements.
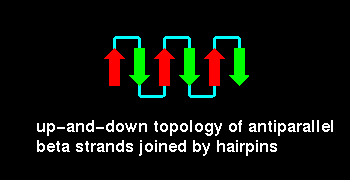
The simplest topology for an antiparallel beta sheet involves loops connecting
adjacent strands, as shown:
The Greek Key topology, named after a pattern that was common on
Greek pottery, is shown below. Three up-and-down beta strands connected by
hairpins are followed by a longer connection to the fourth strand, which
lies adjacent to the first.
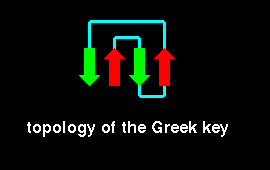
Folds including the Greek key topology have been found to have 5-13 strands.
An example is given below.
plastocyanin (21Kb GIF).
![]() 2plt (71Kb)
[Bbk|BNL|ExP|Waw|Hal]
Here is the crystal structure. Notice that this has a mixed sheet- there
are two parallel pairs of strands. This SCRIPT
renders the molecule as in the diagram.
2plt (71Kb)
[Bbk|BNL|ExP|Waw|Hal]
Here is the crystal structure. Notice that this has a mixed sheet- there
are two parallel pairs of strands. This SCRIPT
renders the molecule as in the diagram.
Gamma-crystallin has two domains each of which is an eight- stranded beta
barrel-type structure composed of two Greek keys:
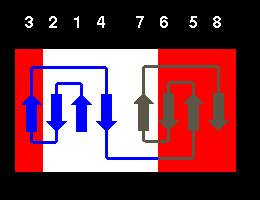
In fact, the structure is more accurately described as consisting of two
beta sheets, one consisting of strands 2,1,4,7 (white) and the other of strands
6,5,8,3 (red) as indicated in the diagram.
![]() This can be seen by examining the crystal structure of gamma-crystallin.
2gcr (136Kb)
[Bbk|BNL|ExP|Waw|Hal]
This SCRIPT displays only the N-terminal domain,
and colours the two sheets as in the diagram above (white and red). To
distinguish the two Greek keys, type the following:
This can be seen by examining the crystal structure of gamma-crystallin.
2gcr (136Kb)
[Bbk|BNL|ExP|Waw|Hal]
This SCRIPT displays only the N-terminal domain,
and colours the two sheets as in the diagram above (white and red). To
distinguish the two Greek keys, type the following:
select 1-39 colour blue select 40-80 colour [90,90,70]
Sequence homology has been found between the two Greek key motifs within each domain, and also between the two domains themselves. The latter homology is higher than the former; this implies that the structure evolved from a single Greek key fold by means of a gene duplication to produce a domain of two Greek keys, followed by a second duplication resulting in two similar domains. This is supported by the fact that in some crystallins each Greek key motif is coded by a different exon, with introns between them.
Richardson(1981) describes the jellyroll fold as being formed by the
addition of an extra "swirl" to a Greek key:
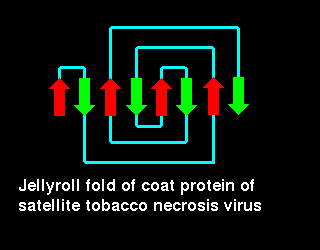
Click here for a diagram illustrating this fold in the coat protein of satellite tobacco necrosis virus (21Kb GIF).
![]() 2stv (158Kb)
[Bbk|BNL|ExP|Waw|Hal]
... SCRIPT to render as in
the diagram
2stv (158Kb)
[Bbk|BNL|ExP|Waw|Hal]
... SCRIPT to render as in
the diagram
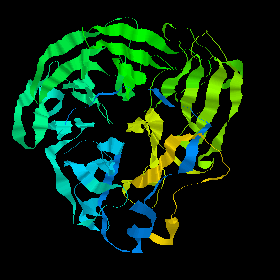
![]() 2bat (304Kb)
[Bbk|BNL|ExP|Waw|Hal]
Click here to examine the crystal structure of one subunit (about 470 residues)
of neuraminidase. One molecule is composed of four of these subunits.
Each is a superbarrel of six four-stranded antiparallel sheets. The
whole structure has a basically up-down topology as shown:
2bat (304Kb)
[Bbk|BNL|ExP|Waw|Hal]
Click here to examine the crystal structure of one subunit (about 470 residues)
of neuraminidase. One molecule is composed of four of these subunits.
Each is a superbarrel of six four-stranded antiparallel sheets. The
whole structure has a basically up-down topology as shown:
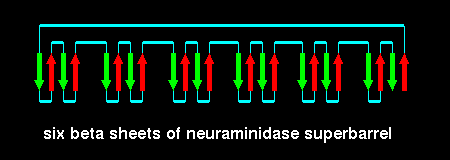
This fold is called a beta propellor; there are six 'blades' in neuraminidase.
![]() Here
are two other examples - how many blades are there in each propellor?:
Here
are two other examples - how many blades are there in each propellor?:
This fold has an approximately 3-fold axis of symmetry.
![]() Examine the crystal structure of erythrina trypsin inhibitor
1tie (114Kb)
[Bbk|BNL|ExP|Waw|Hal]
... SCRIPT
Examine the crystal structure of erythrina trypsin inhibitor
1tie (114Kb)
[Bbk|BNL|ExP|Waw|Hal]
... SCRIPT
This dramatically unusual fold was discovered quite recently. The beta strands
wind round the structure describing a helical topology, as can be seen in
this image of pectate lysase.
![]() 2pec (245Kb)
[Bbk|BNL|ExP|Waw|Hal]
2pec (245Kb)
[Bbk|BNL|ExP|Waw|Hal]

|
|
Last updated 7th April '97