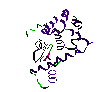 Structure of T 4 Lysozyme showing the residues of 77 and 82
Structure of T 4 Lysozyme showing the residues of 77 and 82
T4 Lysozyme experiment
Glycine and Proline has made the unfolded protein more unstable, both types of mutations have been made in T4 lysozyme. The chosen mutations were Gly77-Ala, which caused an increase in Tm of 1, and Ala82-Pro, which increased Tm by 2.
Hu studied by means of DSC the effects of replacing Ala82 and Ala93 by prolines. Ala 82 is one residue removed from the carboxy terminus of the helix formed by residues 81-91, and since Ala is usually classed as a good helix former and pro as a poor one, the mutation Ala82/Pro might have been expected to result in destabilization.Unexpectedly there have been an apparent stabilization. Therefore the introduction of proline residue decrease the configurational entropy of the unfolded form, producing a stabilization of the native form.It is the same case for Ala93Pro.
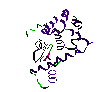 Structure of T 4 Lysozyme showing the residues of 77 and 82
Structure of T 4 Lysozyme showing the residues of 77 and 82  Thermal parameters of T 4 Lysozyme mutants
Thermal parameters of T 4 Lysozyme mutants