Proteoglycans (mucoproteins) are formed of glycosaminoglycans (GAGs) covalently attached to the core proteins.
They are found in all connective tissues, extracellular matrix (ECM) and on the surfaces of many cell types. Proteoglycans are remarkable for their diversity (different cores, different numbers of GAGs with various lenghts and compositions).
Glycosaminoglycans forming the proteoglycans are the most abundant heteropolisaccharides in the body. They are long unbranched molecules containing a repeating disaccharide unit. Usually one sugar is an uronic acid (either D-glucuronic or L-iduronic) and the other is either GlcNAc or GalNAc. One or both sugars contain sulfate groups (the only exception is hyaluronic acid).
GAGs are highly negatively charged what is essential for their
function.
THE SPECIFIC GAGs OF PHYSIOLOGICAL SIGNIFICANCE ARE :
Hyaluronic acid (D-glucuronate + GlcNAc)

Occurence : synovial fluid, ECM of loose connective tissue
Hyaluronic acid is unique among the GAGs because it does not contain
any sulfate and is not found covalently attached to proteins. It forms
non-covalently linked complexes with proteoglycans in the ECM.
Hyaluronic acid polymers are very large (100 - 10,000 kD) and can displace
a large volume of water.
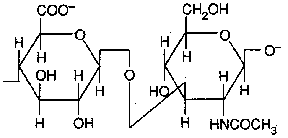
Dermatan sulfate (L-iduronate + GlcNAc sulfate)
Occurence : skin, blood vessels, heart valves
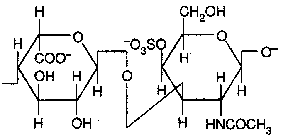
Chondroitin sulfate (D-glucuronate + GalNAc sulfate)
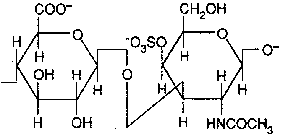
Occurence : cartilage, bone, heart valves ;
It is the most abundant GAG.
Heparin and heparan sulfate (D-glucuronate sulfate + N-sulfo-D-glucosamine)
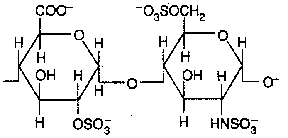
Heparans have less sulfate groups than heparins
Occurence :
Keratan sulfate ( Gal + GlcNAc sulfate)
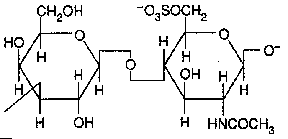
Occurence : cornea, bone, cartilage ;
Keratan sulfates are often aggregated with chondroitin sulfates.
The GAGs extend perpendicular from the core protein in a bottlebrush- like structure.
The linkage of GAGs such as (heparan sulfates and chondroitin sulfates) to the protein core involves a specific trisaccharide linker :
Some forms of keratan sulfates are linked to the protein core through an N-asparaginyl bond.
The protein cores of proteoglycans are rich in Ser and Thr residues which allows multiple GAG attachment.
They perform numerous vital functions within the body.
GAG dependent functions can be divided into two classes: the biophysical and the biochemical.
The biophysical functions depend on the unique properties of GAGs : the ability to fill the space, bind and organize water molecules and repel negatively charged molecules. Because of high viscosity and low compressibility they are ideal for a lubricating fluid in the joints. On the other hand their rigidity provides structural integrity to the cells and allows the cell migration due to providing the passageways between cells.
For example the large quantities of chondroitin sulfate and keratan sulfate found on aggrecan play an important role in the hydration of cartilage. They give the cartilage its gel-like properties and resistance to deformation.
Aggrecan is one of the most important extracellular proteoglycans.
It forms very large aggregates (a single aggregate is one of the largest
macromolecules known ; it can be more than 4 microns long). Aggrecan
molecules are non-covalently bound to the long molecule of hyaluronan
(like bristles to the backbone in a bottlebrush). It is faciliated
by the linking proteins. To each aggrecan core protein multiple chains
of chondroitin sulfate and keratan sulfate are covalently attached through
the trisaccharide linker .
The other, more biochemical functions of
GAGs are mediated by specific binding of GAGs to other macromolecules,
mostly proteins. Proteoglycans participate in cell and tissue
development and physiology.
EXAMPLES OF GAG BINDING PROTEINS :
Secreted proteases and antiproteases
For example antithrombin III (AT III) binds tightly to heparin and certain heparan sulfates (so do its substrates). Thus they control the blood coagulation. In the absence of GAGs AT III inactivates proteases (such as thrombin, factors IXa and XIa) very slowly. In the presence of appropriate GAGs these reactions are accelerated 2000-fold.
GAGs are sufficiently long that both protease and protease inhibitor can bind to the same chain (thus the likelyhood of the two proteins binding to each other is increased enormously). GAGs also affect the protein conformation that contributes to improving AT III binding kinetics.
Polypeptide growth factors
Members of the FGF family, as well as several other growth factors, bind to heparin or heparan sulfate. Binding to endogenous GAGs entraps these molecules in ECM from which they may be later released. GAGs can alter the conformation, proteolytic susceptibility and biological activity of some of these proteins. The bound growth factor is resistant to degradation by extracellular proteases. Active hormone is released by proteolysis of the heparan sulfate chains. It occurs during the tissue growth and remodeling after infection.
ECM proteins
Most of the large, multidomain ECM proteins contain at least one GAG binding site.
For example fibrous collagens (type I, III, V) and fibronectin bind to heparan sulfate chains which are attached to the integral membrane core proteins of cell surface proteoglycans such as syndecan and fibroglycan. Cell surface proteoglycans are thought to anchor cells to matrix fibers.
Cell - cell adhesion molecules
 When the level of hyaluronan
is lower (e.g. because of digesting by hyaluronidase), there is ceesation
of cell movement and initiation of cell- cell attachment.
When the level of hyaluronan
is lower (e.g. because of digesting by hyaluronidase), there is ceesation
of cell movement and initiation of cell- cell attachment.
Go to :