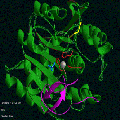
 Try this view of
Carboxypeptidase A. Notice how the Zn2+ ion
(grey sphere in the center) is surrounded by three side chains (in red).
The magenta ribbon structure is the potato inhibitor of the enzyme.
Try this view of
Carboxypeptidase A. Notice how the Zn2+ ion
(grey sphere in the center) is surrounded by three side chains (in red).
The magenta ribbon structure is the potato inhibitor of the enzyme. Proteases prune other proteins by selectively cleaving peptide bonds. Metalloproteases bind a metal ion such as Zn2+ in the active site. Some of the most widely studied metalloproteases have been digestive enzymes such as carboxypeptidases A and B and thermolysin.
Traditionally, these enzymes are categorized as being either "exopeptidases," which catalyzes the step wise removal of single residues from the carboxyl terminus of a polypeptide substrate, and "endopeptidases" which catalyze the hydrolysis of non-terminal peptide bonds, especially those with hydrophobic residues. These distinctions are not particularly useful at the molecular level.
Usually the Zn2+ ion serves to coordinate two to four side chains. In thermolysin and carboxypeptidase the Zn2+ atom is bound by interactions with the imidazole (polar rings) side chains of two His residues and with the carboxyl side chain of a Glu residue. Importantly, the Zn2+ is also coordinated by a water molecule which plays a crucial role in the catalytic activity of each enzyme.
 Try this view of
Carboxypeptidase A. Notice how the Zn2+ ion
(grey sphere in the center) is surrounded by three side chains (in red).
The magenta ribbon structure is the potato inhibitor of the enzyme.
Try this view of
Carboxypeptidase A. Notice how the Zn2+ ion
(grey sphere in the center) is surrounded by three side chains (in red).
The magenta ribbon structure is the potato inhibitor of the enzyme.
The above is actually the item "4CPA_carboxypeptidase-A_1.gif" in the
There are 14 thermolysin structures available at the PDB. To see what is available there, go to the PDB (by clicking on the word), and click on PDB Browser. Enter thermolysin under Compound. Then Send Request and when a List of ID Codes appears, choose one and Fetch... You can download the pdb file, view the molecule by with a local client (such as Rasmol), or for some molecules, view a related GIF file, such as what we saw above.
Daniel Barsky (barsky@ca.sandia.gov)