Many enzymes, including all those involved in glycolysis , are alpha/beta structures. Most alpha/beta proteins are cytosolic.
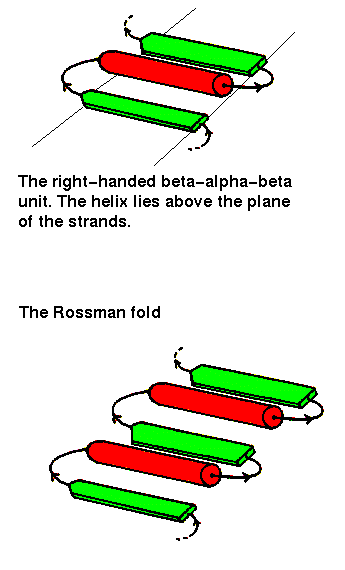
The beta-alpha-beta unit has already been described in a previous chapter . This motif is always right-handed. In alpha/beta structures, there is a repetition of this arrangement, giving a beta-alpha-beta-alpha.....etc sequence. The beta strands are parallel and hydrogen bonded to each other, while the alpha helices are all parallel to each other, and are anitparallel to the strands. Thus the helices form a layer packing against the sheet.
The beta-alpha-beta-alpha-beta subunit, often present in nucleotide-binding proteins, is named the Rossman Fold, after Michael Rossman (Rao and Rossman,1973).

Richardson (1981) names the alpha/beta structures "parallel alpha/beta domains", to denote the fact that each of the 2 secondary structures forms a parallel arrangement. Note that there is no obvious reason why one would not expect to find "parallel all alpha" (alpha-alpha-alpha subunit) folds, or "parallel all beta" (beta-beta-beta) folds in equally large numbers, but these do not occur. However, the marked tendency for helices to pack aligned with sheets has been explained by the "complementary twist" model (Chothia et al. , 1977). The right-handed twist of beta sheets and the right-handed twist of the row of every 4th residue of the helices (the "i+4" ridges"- see section on helix-helix packing in the page on all alpha folds) mean that the two have complementary surfaces when aligned. This model is supported by the observation that approximately 90% of the helix residues which interface with a sheet are indeed a multiple of 4 residues apart. Helices packing side by side on a sheet would have helices rotated with respect to each other, due to the sheet twist; the observed interhelical angle is in agreement with this model in 80% of cases. In the other cases the helices are splayed from the sheet, with only one end in contact.
 The
structure of the remarkable placental ribonuclease inhibitor
(Kobe, B. & Diesenhofer, J. (1993 ) Nature V.366, 751- )takes the
concept of the repeating alpha/beta unit to extremes. It is a cytosolic
protein that binds extremely strongly to any ribonuclease that may leak into the
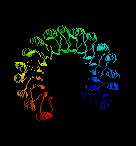
cytosol. Take a look (pdb, gif)
and you'll see the 17-stranded parallel beta sheet curved into an open
horseshoe shape, with 16 alpha-helices packed against the outer surface.
It doesn't form a barrel although it looks as though it should. The strands are only
very slightly slanted, being nearly parallel to the central `axis'.
The
structure of the remarkable placental ribonuclease inhibitor
(Kobe, B. & Diesenhofer, J. (1993 ) Nature V.366, 751- )takes the
concept of the repeating alpha/beta unit to extremes. It is a cytosolic
protein that binds extremely strongly to any ribonuclease that may leak into the
cytosol. Take a look (pdb, gif)
and you'll see the 17-stranded parallel beta sheet curved into an open
horseshoe shape, with 16 alpha-helices packed against the outer surface.
It doesn't form a barrel although it looks as though it should. The strands are only
very slightly slanted, being nearly parallel to the central `axis'.
If the first strand hydrogen
bonds to the last, then the structure closes on itself forming a barrel-like
structure. This is shown in the
picture of triose phosphate isomerase:

Note that the "staves" of the barrel are slanted, due to the twist of the beta sheet. Also notice that there are effectively four layers to this structure. The direction of the sheet does not change (it is anticlockwise in the diagram). Such a structure may therefore be described as singly wound.
In a structure which is open rather than closed like the barrel, helices would be situated on only one side of the beta sheet if the sheet direction did not reverse (see previous figure. ). Therefore open alpha/beta structures must be doubly wound to cover both sides of the sheet.
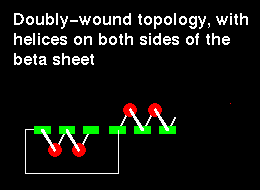
The chain starts in the middle of the sheet and travels outwards, then returns to the centre via a loop and travels outwards to the opposite edge:
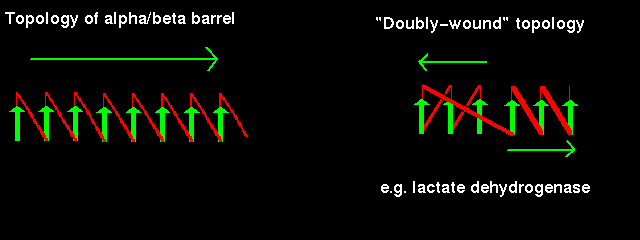
Doubly-wound topologies where the sheet begins at the edge and works inwards are rarely observed.
Click here for a diagram illustrating the doubly wound fold of the first domain of lactate dehydrogenase.
Click here for some examples of variations on the doubly-wound alpha-beta topology.
Back to Main PPS Index
J. Walshaw