Last modified 12th April '95 © Birkbeck College 1995
Back to main PPS Index
Back to Protein Folds Index
All-Alpha Topologies
With MAGE installed, study this
Kinemage
The Lone Helix
There are a number of examples of small proteins (or peptides) which
consist of little more than a single helix. A striking example is
 glucagon, a hormone involved
in regulating sugar metabolism in mammals (as does insulin).
glucagon, a hormone involved
in regulating sugar metabolism in mammals (as does insulin).
The Helix-turn-helix motif
Different variations of this supersecondary structure have been introduced in
a previous chapter . The simplest
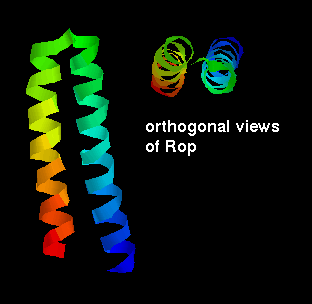
packing arrangement of a domain of two helices is for them to lie antiparallel,
connected by a short loop. This constitutes the structure of the small (63
residue) RNA-binding protein
Rop
, which is found in certain plasmids
(small circular molecules of double-stranded DNA occurring in bacteria and
yeast) and involved in their replication. There is a slight twist in the
arrangement as shown.
The Four-helix Bundle
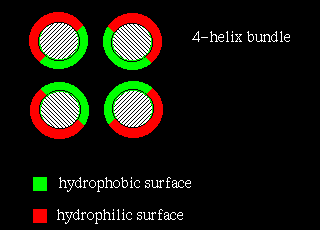
The four-helix bundle is found in a number of different proteins. In many cases
the helices part of a single polypeptide chain, connected to each other by three
loops. However, the Rop molecule is in fact a dimer of two of the two-helix
units shown above.
In four-helix-bundle proteins the interfaces between the helices consist
mostly of hydrophobic residues
while polar side chains on the exposed surfaces interact with the aqueous
environment, as indicated below:
 Compare this with the arrangement of residues that
would be expected in a membrane-spanning helical domain, which has
previously been indicated.
The central helices of the
photosynthetic reaction centre in fact are arranged similar to the four-
helix bundle.
Compare this with the arrangement of residues that
would be expected in a membrane-spanning helical domain, which has
previously been indicated.
The central helices of the
photosynthetic reaction centre in fact are arranged similar to the four-
helix bundle.
Other examples exhibit a much more open packing arrangement, as in the steroid-binding
proteins uteroglobin, and Clara cell 17kDa protein
(gif, pdb)
Topologies
The four helices may be arranged in a simple up-and-down topology, as indicated.
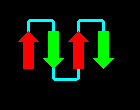 Click here for a
diagram of
myohemerythrin, which
has this fold (others are
cytochrome c',
cytochrome b-562,
which have two molecules in the asymmetric unit).
Click here for a
diagram of
myohemerythrin, which
has this fold (others are
cytochrome c',
cytochrome b-562,
which have two molecules in the asymmetric unit).
A more complex arrangement is possible:
 Click here for the
four-helix bundle topology of
ferritin.
Click here for the
four-helix bundle topology of
ferritin.
The ligand-binding sites of these proteins occur between the alpha helices.
The ligands can be seen by selecting the structures listed above and choosing
the Display:sticks and Colour:chain options, e.g. below is a diagram of
cytochrome C' indicating the haem group, which is sited between the first and
fourth helices.
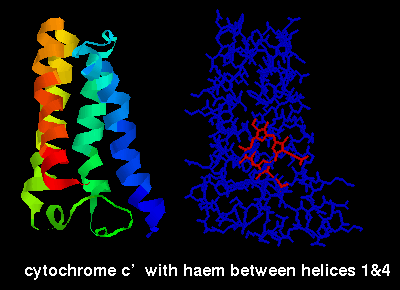
Cytokines
A number of cytokines consist of four alpha helices in a bundle. Click
here for a diagram of Interleukin-2, human Growth Hormone, Granulocyte-macrophage
colony-stimulating factor (GM-CSF) and Interleukin-4, by Manuel Peitsch of
Glaxo Geneva.
Also examine Simon Brocklehurst's
page on cytokines.
Alpha domains which bind DNA
Transcription factors are proteins which bind to control regions
of DNA. These regions are "upstream" of the structural gene (the sequence
which actually codes for a protein) whose transcription they regulate.
Transcription factors have a DNA-binding domain and a domain that activates
transcription.
The RNA-binding two-helix protein Rop has already been mentioned. A three-helix
bundle forms the basis of a DNA-binding domain which occurs in a number of
proteins- for example homeodomain proteins. Examine the crystal structure
of engrailed
homeodomain binding to DNA, and
three
different
diagrams courtesy of Manuel Peitsch.
 Click here for the structure of the cro
repressor from phage 434.
Click here for the structure of the cro
repressor from phage 434.
Also refer to the GCN4 transcription factor leucine zipper described in Antti Iivanainen's section
on coiled coils.
Globins
The globin fold usually consists of eight alpha helices (e.g.
myoglobin). The two helices at the end
of the chain are antiparallel, forming a helix-turn-helix motif, but the
remainder of the fold does not include any characterized supersecondary
structures. These helices pack against each other with larger angles, around
50 °, between them than occurs between antiparallel helices (approximately
20°). See the section below on helix-helix packing. Jane Richardson (1981)
describes the globin fold as a "Greek key helix bundle", due to the topological
similarity with the Greek key arrangement of antiparallel beta-sheets (see
section on all beta topologies).
In all, fifty-six categories of
"mostly alpha"
folds are listed in the
Structural Classification of Proteins database. A number of the entries
have links to appropriate diagrams by Manuel Peitsch. The
CATH Protein
Structure Classification Database" at UCL lists 9 orthogonal, and 3
aligned topologies of
"Mainly
Alpha" structures.
Helix-helix packing
When alpha-helices pack against each other, the side-chains in their
interface are buried.
The two interface areas should have complementary surfaces. The surface
of an alpha-helix can
be thought of as consisting of grooves and ridges, like a screw thread :
for instance, the side
chains of every 4th residue form a ridge (because there are 3.6 residues
per turn). The
direction of this ridge is 26° from the direction of the helix axis.
Therefore if 2 helices pack
such that such a ridge from each fits into the other's groove, the expected
angle between the
two is 52°. In fact, in the distribution of this angle between packed alpha-helices,
there is a sharp
peak at 50°. Besides the type of ridge described, ridges can be formed by
other staggerings
of residues, such as every 3rd residue, or indeed every residue. Which ridges are used for
packing depends on the size and conformations of the side chains at these relative positions.
The "i+4" ridge is believed to be the most common because residues at every 4th position
have side-chains which are more closely aligned than in "i+3" or "i+1" ridges
as indicated below.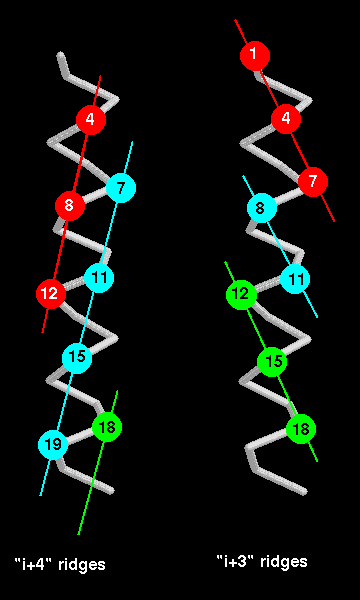 Again, refer to Chothia (1984)
Again, refer to Chothia (1984)
Two
other types of packing do occur, however : between an "i+4" ridge and an "i+3"
ridge (there
is an angle of 23° between the 2 helix axes) and between an "i+4" and an
"i+1" ridge (the
helices are 105° apart). The
"ridges and grooves"
model does not describe all the helix-helix packings, as there are examples
with unusual
interaxial angles. For instance in the globin fold a pair of helices
(B and E) pack such that their ridges
cross each other, by means of a notch formed at a pair of glycine residues.
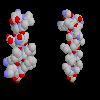 Click here for a diagram of
the notch in the ridges of helices B and H, and
Click here for a diagram of
the notch in the ridges of helices B and H, and
 click here for a slice
through a space-filling model of the two helices packing against each other.
click here for a slice
through a space-filling model of the two helices packing against each other.
The interaxial distance between packed helices varies from 6.8-12.0Å,
the mean being
9.4 Å;the mean interpenetration of atoms at the interface is 2.3Å.
Therefore it is mainly side
chains which make the contacts between the helices.
Other Distinctive All-alpha proteins include :-
Back to Main PPS Index
J. Walshaw & Alan Mills
 Compare this with the arrangement of residues that
would be expected in a membrane-spanning helical domain, which has
previously been indicated.
The central helices of the
photosynthetic reaction centre in fact are arranged similar to the four-
helix bundle.
Compare this with the arrangement of residues that
would be expected in a membrane-spanning helical domain, which has
previously been indicated.
The central helices of the
photosynthetic reaction centre in fact are arranged similar to the four-
helix bundle.
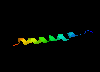 glucagon, a hormone involved
in regulating sugar metabolism in mammals (as does insulin).
glucagon, a hormone involved
in regulating sugar metabolism in mammals (as does insulin).

 Click here for a
diagram of
myohemerythrin, which
has this fold (others are
cytochrome c',
cytochrome b-562,
which have two molecules in the asymmetric unit).
Click here for a
diagram of
myohemerythrin, which
has this fold (others are
cytochrome c',
cytochrome b-562,
which have two molecules in the asymmetric unit).
 Click here
Click here 
 Click here
Click here Again, refer to Chothia (1984)
Again, refer to Chothia (1984)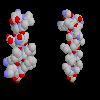 Click here
Click here  click here
click here