Back to main Molecular Forces index

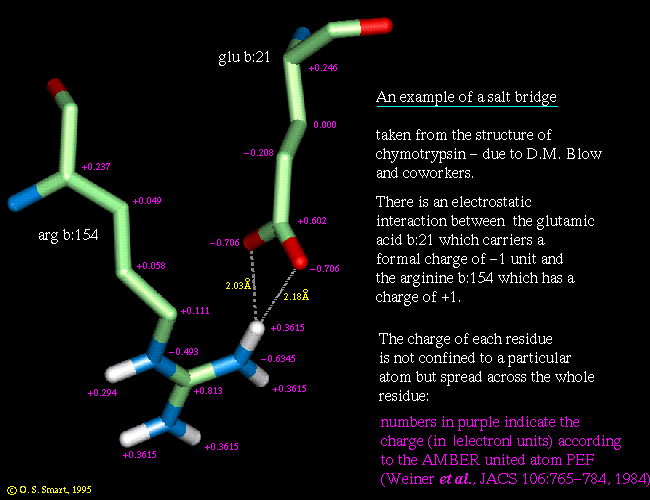
Note that oxygen atoms are shown in red, nitrogens are in blue, hydrogens in white and carbon atoms are in light green. Only the hydrogen atoms bonded to an oxygen or nitrogen are shown. This is fairly routine approximation called the "united-atom" or "extended-atom" representation, which is an option in many potential energy functions. The hydrogen atoms which should be attached to carbons are not explicitly represented but instead a small adjustment is made to the LINK NEEDED van der Waals parameters of the carbon atom.