 Click here for a gif showing the
Tyr13/Tyr17 aromatic interaction in barnase and link to pdb.
Click here for a gif showing the
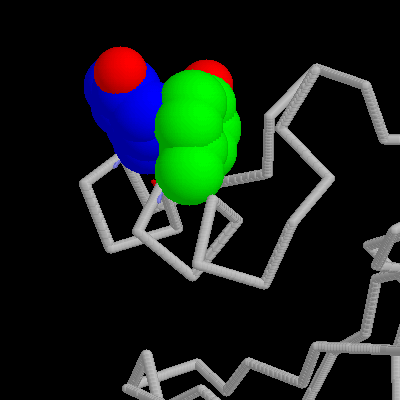
Tyr13/Tyr17 aromatic interaction in barnase and link to pdb.About 60% of the aromatic side chains (Phe, Tyr, and Trp), found in proteins
are involved in aromatic pairings. Studies with model compounds suggest
that the optimal geometry is perpendicular, such that the partially positively
charged hydrogens on the edge of one ring can interact favourably with the
pi electrons and partially negatively charged carbons of the other. From
these observations, it might be expected that such interactions make a contribution
to protein stability. This has been tested on the solvent exposed Tyr13
/ Tyr17 pair on the surface of barnase (Serrano
et al., 1991). Tyr13 and Tyr17 were mutated to both Ala and Phe.
 Click here for a gif showing the
Tyr13/Tyr17 aromatic interaction in barnase and link to pdb.
Click here for a gif showing the
Tyr13/Tyr17 aromatic interaction in barnase and link to pdb.
Mutation of the Tyr13 or Tyr17 to Phe leads to a decrease in stability of 0.30 or 0.41 kcal/mol, and 0.61 kcal/mol in the double mutant. As both hydroxyl groups are exposed to solvent (as they would be in the unfolded state), it is unclear as to the source of this small destabilization. However, mutation to the double alanine mutant leads to a decrease in stability of 4.6 kcal/mol. Most of this destabilization can be explained in terms of the loss of interactions between the tyrosine residues and the rest of the protein; however, analysis of the data using thermodynamic cycles (Carter, 1984; Horovitz & Fersht, 1990) indicates that the interaction energy between the two aromatic groups contributes only 1.3 kcal/mol to the protein stability. This is only very slightly higher than the stabilization expected from the hydrophobic contribution from burying surface area between them. In this case, therefore, there is little apparent extra stabilization due to the aromatic pair.
![]() Salt Bridges
Salt Bridges ![]() Metal Binding
Metal Binding![]() Beginning
Beginning