Back to looking at bond potential
Back to main Molecular Forces index
Back to main PPS course Index
The morse potential is a convenient model for the potential energy
of a diatomic molecule. It is much used in spectroscopic applications
(reference to Dwek and Campbell needed) as it is possible
to solve Schrödinger equation for this system (see Atkins).
The functional form of the potential is: 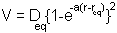
Where 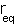 is the equilibrium bond
length and
is the equilibrium bond
length and 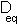 the potential energy
for bond formation and "a" a parameter controlling the
width of the potential well. If we plot the function it looks
like:
the potential energy
for bond formation and "a" a parameter controlling the
width of the potential well. If we plot the function it looks
like:
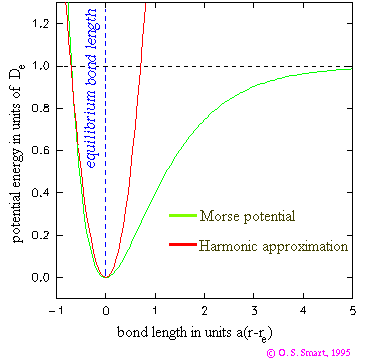
Shown as red line is a harmonic fit to the Morse potential around the equilibrium position: showing how, in this case, Hooke's law can be used as a useful approximation.