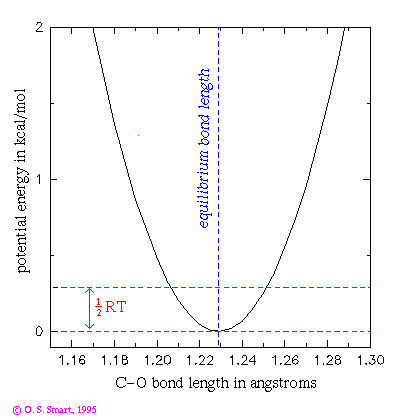

This graph shows the potential energy for a C=O bond using a harmonic potential:
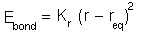 ,
,
with the parameters given in the AMBER potential
energy function. The bond constant K_r is 570 kcal/(molÅ^2)
and the equilibrium bond length is 1.229 Å
(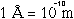 ) .
The dashed red lines indicates an energy of 0.29 kcal/mol which is equal
to 1/2RT at a temperature of 300K. This is the energy that an individual
degree of freedom can expect at this temperature and indicates that a this
bond could be expect to be undergoing vibrations of the order of 0.03 Å
at room temperature.
) .
The dashed red lines indicates an energy of 0.29 kcal/mol which is equal
to 1/2RT at a temperature of 300K. This is the energy that an individual
degree of freedom can expect at this temperature and indicates that a this
bond could be expect to be undergoing vibrations of the order of 0.03 Å
at room temperature.
Oliver Smart
©O.S. Smart 1995, all rights reserved
Back to main Molecular Forces index
Back to main PPS course Index