

|
|
|
|
As can be seen below there are a number of small aliphatic side chains containing polar groups which cannot ionise readily. Serine and threonine possess hydroxyl groups in their side chains and as these polar groups are close to the main chain they can form hydrogen bonds with it. This can influence the local conformation of the polypeptide, indeed residues such as serine and asparagine are known to adopt conformations which most other amino acids cannot. The amino acids asparagine and glutamine posses amide groups in their side chains which are usually hydrogen-bonded whenever they occur in the interior of a protein. Here is a pdb-file for RasMol.
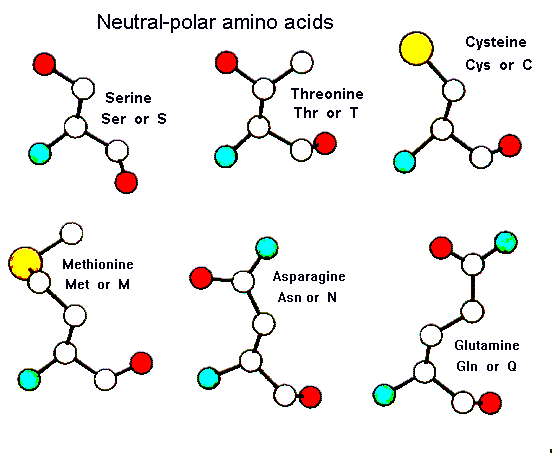
There are two sulphur containing amino acids (cysteine and methionine) which are largely non-polar in character. Indeed methionine could reasonably be classed as a hydrophobic residue as it is nearly always associated with the hydrophobic cores of proteins. Cysteine has the unique property of being able to form a covalent cross-link with another cysteine residue elsewhere in the protein. These disulphide bridges involve -S-S- bonds being formed between spatially adjacent cysteine residues. The great cohesive strength of certain keratin proteins such as tortoise shell can be attributed to the large number of disulphide bridges they contain. Disulphide bridges are sensitive to reducing agents which convert the two sulphur atoms back to their original -S-H form. Cysteines frequently occur at metal binding sites as their sulphur atoms can form dative covalent bonds with certain metal ions. Serine and cysteine frequently have a catalytic role in enzyme active sites.
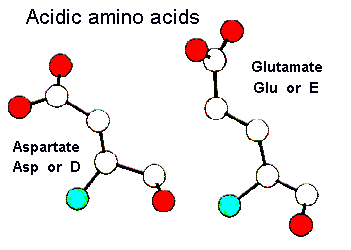
Two amino acids, aspartate and glutamate, have carboxyl side chains and are therefore negatively charged at physiological pH (around neutral). The strongly polar nature of these residues means that they are most often found on the surface of globular proteins where they can interact favourably with solvent molecules. These residues can also take part in electrostatic interactions with positively charged basic amino acids. Aspartate and glutamate also can take on catalytic roles in the active sites of enzymes and are well known for their metal ion binding abilities. Here is a pdb-file for RasMol.
Last updated 9th Oct'96