

|
|
|
Haemoglobin, like myoglobin is an oxygen-binding protein. Whereas myoglobin
exists as a monomer, haemoglobin is a tetramer: each of the four subunits
is similar in terms of fold to myoglobin. Haemoglobin is a hetero-tetramer,
consisting of two alpha subunits and two beta subunits (a "dimer of dimers"),
the alpha subunits (141 residues in human haemoglobin) and beta subunits
(146) being homologous. This form is called haemoglobin A.
The binding of oxygen by haemoglobin is cooperative ; the protein cannot be considered in terms of four independently oxygen-binding subunits. As haemoglobin binds successive oxygens, the oxygen affinity of the subunits increases. The affinity for the fourth oxygen to bind is approximately 300 times that for the first. This cannot be explained by four independent subunits with intrinsically different affinities, as if this were the case, then oxygen would bind to the subunit with the highest intrinsic affinity; the affinity of the haemoglobin molecule would subsequently decrease rather than increase.
This behaviour results in an oxygen dissociation curve (a plot of saturation of oxygen-binding sites vs partial pressure of oxygen) that is sigmoidal, rather than hyperbolic. Hyperbolic curves are exhibited by monomeric molecules such as myoglobin, the monomeric alpha chain, and other non-cooperative molecules such as haemoglobin H (see below).

The significance of the sigmoidal curve is that it means that haemoglobin A becomes highly saturated at high oxygen partial pressures, and releases a significant amount of oxygen at pressures which are fairly low, but not extremely so. Contrast this to the hyperbolic myoglobin curve, where even at the low partial pressure of 20 mmHg, the oxygen-carrier is still almost totally saturated. 20mmHg is in fact the partial oxygen pressure in the capillaries of active muscles. Aterial pressure is 100mmHg. Haemoglobin A is 50% saturated at 26mmHg, while 50% saturation of myoglobin occurs at only 1mmHg.
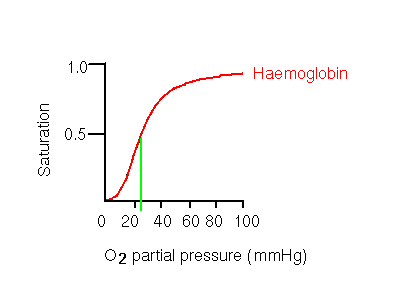
Therefore, a cooperative binding mechanism is more efficient at collecting oxygen where it is in high concentration, and supplying it where it is needed. Tissues need be only relatively mildly deficient in oxygen for haemoglobin to give up a much more significant amount compared to a non-cooperative system such as myoglobin.
Haemoglobin may be considered to exist in two states: the tense (T) state, and the relaxed (R) state. The T form corresponds to the quaternary structure of deoxyhaemoglobin, and R to that of oxyhaemoglobin. The binding of a molecule of oxygen to a haemoglobin subunit stabilizes the R state, and thereby increases the affinity of remaining deoxygenated subunits for oxygen.
The cooperative oxygen-binding properties of haemoglobin rely on specific quaternary interactions between the subunits. In isolation, the beta subunits, form a homo-tetramer, (called haemoglobin H), which lacks the particular subunit interactions of haemoglobin A. Haemoglobin H therefore does not exhibit any cooperativity in its oxygen binding.
The allosteric mechanisms of haemoglobin A enables the affinity for oxygen to be adjusted by interactions with a number of reagents.
The oxygen affinity of haemoglobin is modified by several factors:
The various mechanisms result in haemoglobin having a lower affinity for oxygen than myoglobin.

|
|
|
Last updated 16th April '97