|
Mosaic Proteins
This page contains a number of links to diagrams by
Peer Bork and
Annalisa
Pastore of the European Molecular
Biology Laboratory, Heidelberg, Germany.
Multi-domain proteins were introduced in a previous section . Domains in such a protein may be considered to be connected units which are independent in terms of their structure, function and folding behaviour. Examination of the database of known amino-acid sequences has revealed that certain sequences are repeated many times throughout a number of different proteins. Such a sequence has a similar secondary and tertiary structure in each case, and is termed a module . The consensus sequence of the 'EG' (or 'G') module, which resembles epidermal growth factor, is shown below.
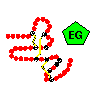 Click
here for figure. Here is the
tertiary
structure.
Click
here for figure. Here is the
tertiary
structure.
This results in a 'construction set' of a relatively small number of modules, from which many different proteins, called mosaic proteins, are formed, with varying lengths of polypeptide chain connecting the modules. Some proteins are formed from one or a few different modules repeated many times. There is a nomenclature of protein modules available from Peer Bork of Chris Sander's group at EMBL. Click here for a diagram of selected mosaic proteins containing the egf-like EG module, and here for those of the extracellular matrix. Illustrations of the modular nature of other types of proteins can be found in Peer Bork's list .
This phenomenon makes possible the prediction of the conformation of many polypeptides whose structures could not be deduced by other means. Once the structure of a module in isolation has been determined, for example by NMR spectroscopy, then the structure of homologous modules can be confidently predicted. Many mosaic proteins are constituents of the extracellular matrix or are membrane proteins, whose structures are difficult to determine by crystallographic methods.
The segmental nature of these proteins indicates that the different modules have had different origins during the evolution of the genome. Many modules correspond to one exon (expressed sequence in a gene). It appears that mosaic proteins are the result of the duplication of exons and their shuffling between different genes. This is more likely to occur successfully in eukaryotic cells, because of the occurrence of introns (intervening, ie unexpressed sequences), in which cleavage and splicing can occur. Mosaic proteins are particularly abundant in vertebrates. In prokaryotes, gene fusion must be precise in order to preserve the reading frame of the nucleic acid. In some bacteria the enzymes involved in trp synthesis are all encoded in different genes, whereas E. coli has two bifunctional polypeptides, each the result of the fusion of two genes.
A simple example of gene duplication occurs in the ferredoxins, where the two halves of the chain have a sequence consensus and a similar conformation. Compare the two halves of the sequence of ferredoxin in Peptococcus aerogenes:
1 10 20
A Y V I N D S C I A C G A C K P E C P V N I I Q G S
I Y A I D A D S C I D C G S C A S V C P V G A P N P E D
30 40 50
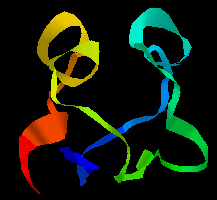 Here is the tertiary structure; the symmetry of the
two halves is apparent. Note that the C-terminal regions of each half of
the sequence would not be expected to show any homology, as the former forms
the loop between the two halves.
Here is the tertiary structure; the symmetry of the
two halves is apparent. Note that the C-terminal regions of each half of
the sequence would not be expected to show any homology, as the former forms
the loop between the two halves.
![]() P. aerogenes ferredoxin 1fdx
(41Kb)[Bbk|BNL|ExP|Waw|Hal]
for the structure in RasMol (select Display:backbone or ribbons, Colours:group).
P. aerogenes ferredoxin 1fdx
(41Kb)[Bbk|BNL|ExP|Waw|Hal]
for the structure in RasMol (select Display:backbone or ribbons, Colours:group).
The tertiary structure of the F3 type module, first found in fibronectin, is shown below. Note that the orientation of the N- and C-terminii of the chain would allow a succession of these modules to be joined together "bead-like",as in fibronectin. This 94-residue domain is of the immunoglobulin beta-sandwich type, consisting of 7 strands forming two sheets in "Greek-key" arrangement (see section on all-beta protein folds).
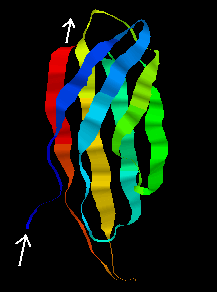
![]() Examine the structure, which was determined by NMR, by clicking here
1ttf
(4.1Mb)[Bbk|BNL|ExP|Waw|Hal].
3D images of the tertiary structures of this
fibronectin
type-III module, and
other selected
modules have been prepared by Annalisa Pastore of EMBL.
Examine the structure, which was determined by NMR, by clicking here
1ttf
(4.1Mb)[Bbk|BNL|ExP|Waw|Hal].
3D images of the tertiary structures of this
fibronectin
type-III module, and
other selected
modules have been prepared by Annalisa Pastore of EMBL.
The average NMR structure of the F1 module from Tissue-plasminogen activator (tPA) is shown below. This is a smaller (50-residue) all-beta domain; it is involved in binding to fibrin (see below).
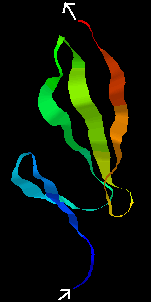
![]() Click here 1tpn
(1.9Mb)[Bbk|BNL|ExP|Waw|Hal]
for the RasMol structure, where 2 disulphide bridges can be seen.
Click here 1tpn
(1.9Mb)[Bbk|BNL|ExP|Waw|Hal]
for the RasMol structure, where 2 disulphide bridges can be seen.
The kringle (KR) domain from the same protein, again determined by NMR methods, is shown below.
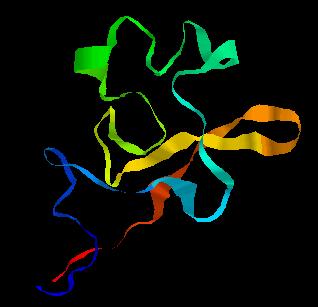 The kringle fold is rich in disulphide bonds (three
are visible in the
The kringle fold is rich in disulphide bonds (three
are visible in the
![]() NMR
structure 1pk2
(116Kb)[Bbk|BNL|ExP|Waw|Hal])
and is composed mostly of beta strands but there is one helix. This same
tPA kringle domain has also been crystallized and the structure can be seen
here 1tpk
(182Kb)[Bbk|BNL|ExP|Waw|Hal](there
are 3 kringle domains in the asymmetric unit). Here is another representation
of the
kringle tertiary structure.
NMR
structure 1pk2
(116Kb)[Bbk|BNL|ExP|Waw|Hal])
and is composed mostly of beta strands but there is one helix. This same
tPA kringle domain has also been crystallized and the structure can be seen
here 1tpk
(182Kb)[Bbk|BNL|ExP|Waw|Hal](there
are 3 kringle domains in the asymmetric unit). Here is another representation
of the
kringle tertiary structure.
There are in fact 2 KR domains in t-PA. The tertiary structure is represented below.
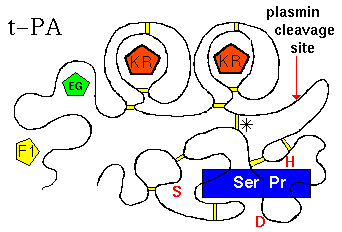
(Diagram adapted from Kreis and Vale, 1993)
(Click here for the modular composition of t-PA, u-PA and plasminogen; by Peer Bork).
The largest, C-terminal domain is the functional, catalytic module: the serine
protease (Ser Pr)domain. The function of t-PA is to cleave a particular
peptide bond in plasminogen, forming plasmin (which is itself a serine protease).
The activity of the enzyme is markedly increased by binding to fibrin, which
is effected by the F1 domain, and the C-terminal kringle domain. Note that
the serine protease domain is connected to the others (F1, EG, KR, KR)
by a disulphide bridge (marked '*'). In fact if the chain is cleaved at the
indicated site by plasmin, the activity of the resulting 2-chain enzyme is
increased (positive feedback mechanism). t-PA is inactivated by Plasminogen
Activator Inhibitors 1 and 2 (PAI-1, PAI-2), which involves residues
Lys-296 and Arg-304 of the serine protease domain, but the C-terminal kringle
may also be involved in the initial binding of PAI-1.The residues of the
catalytic triad of the active site of the Ser Pr domain are indicated in
red (Ser,Asp,His).
The Urokinase-Type Plasminogen Activator (u-PA) functions is a similar
fashion.
Dorin-Bogdan Borza provides this material on Histidine-rich Glycoprotein (HRG), at the University of Missouri-Kansas City.
Alan Mills describes the selectins, one of four major families of membrane-associated cell adhesion proteins.
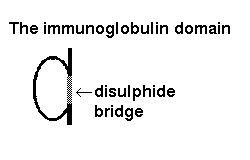
The immunoglobulin structural module is one of the best known examples of exon shuffling and duplication. It is present in a wide variety of proteins on the surfaces of cells. The fold consists of 9 antiparallel beta strands, forming two beta-sheets in a 'sandwich'. This kind of architecture is described in Section 9.
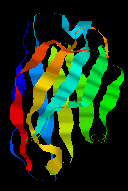 Click
here for a larger picture of the structure of the domain, which indicates
the disulphide bridge which links the two sheets of the "sandwich". See also
Annalisa Pastore's diagram of
immunoglobulin
tertiary structure. The domain may be represented like this
Click
here for a larger picture of the structure of the domain, which indicates
the disulphide bridge which links the two sheets of the "sandwich". See also
Annalisa Pastore's diagram of
immunoglobulin
tertiary structure. The domain may be represented like this
An antibody such as IgM is composed of two heavy chains, each of which consists of 4 immunoglobulin domains, and two light chains of 2 domains each.
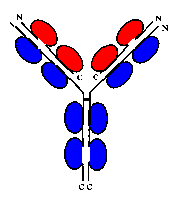 Click
here for a diagram of an antibody. On each of
the 4 chains, the N-terminal domains are known as variable domains, as the
loops connecting the beta strands are subject to exon shuffling, creating
the diversity of antibodies capable of binding to a variety of antigens.
Note however that in any one antibody molecule, the two light chains are
identical, and the two heavy chains are identical, giving two identical
antigen-binding sites at the end of each 'arm'.
Click
here for a diagram of an antibody. On each of
the 4 chains, the N-terminal domains are known as variable domains, as the
loops connecting the beta strands are subject to exon shuffling, creating
the diversity of antibodies capable of binding to a variety of antigens.
Note however that in any one antibody molecule, the two light chains are
identical, and the two heavy chains are identical, giving two identical
antigen-binding sites at the end of each 'arm'.
![]() Human immunoglobulin G1 with a hinge deletion
1mco (443Kb)
[Bbk|BNL|ExP|Waw|Hal]
The asymmetric unit consists of one light and one heavy chain. A symmetry
operation needs to be applied to obtain a complete antibody molecule (albeit
with some disulphide bonds broken). This has been done by PPS Consultant
Will Pitt- click for the resulting structure file.
Human immunoglobulin G1 with a hinge deletion
1mco (443Kb)
[Bbk|BNL|ExP|Waw|Hal]
The asymmetric unit consists of one light and one heavy chain. A symmetry
operation needs to be applied to obtain a complete antibody molecule (albeit
with some disulphide bonds broken). This has been done by PPS Consultant
Will Pitt- click for the resulting structure file.
![]() You can obtain the
coordinate file
of a model of an entire human IgG1 molecule, on Eric
Martz' RasMol Home
Page, at the University of Massechusetts Amherst. The file was provided
by Eduardo Padlan of the National Institutes of Health, Bethesda.
You can obtain the
coordinate file
of a model of an entire human IgG1 molecule, on Eric
Martz' RasMol Home
Page, at the University of Massechusetts Amherst. The file was provided
by Eduardo Padlan of the National Institutes of Health, Bethesda.
Papain and pepsin both cleave the heavy chains between the 2nd and 3rd domains.
Papain cleaves on the N-terminal side of the two disulphide bonds linking
the two heavy chains. This gives two separate,identical Fab fragments
(The 2 C-terminal domains of the heavy chains forming an Fc
fragment).
![]() Click here for the crystal structure of an Fab fragment.
2fbj
(321Kb)[Bbk|BNL|ExP|Waw|Hal]
Click here for the crystal structure of an Fab fragment.
2fbj
(321Kb)[Bbk|BNL|ExP|Waw|Hal]

![]() Here is the crystal structure of a fragment of Fab
1igm
(172Kb)[Bbk|BNL|ExP|Waw|Hal]
consisting of one light chain domain and one heavy chain domain.
Here is the crystal structure of a fragment of Fab
1igm
(172Kb)[Bbk|BNL|ExP|Waw|Hal]
consisting of one light chain domain and one heavy chain domain.
Pepsin on the other hand cleaves on the C-terminal side of the disulphide
linkages, giving a single F(ab')2 fragment, consisting of 2 F(ab')
fragments (slightly longer than an Fab) linked by the two disulphide bonds
(and the Fc region is cleaved into several subfragments):
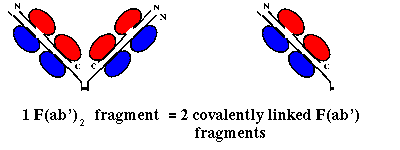
![]() Here is the crystal structure of a F(ab')
1igf
(559Kb)[Bbk|BNL|ExP|Waw|Hal]
fragment. There are two F(ab') fragments in the asymmetric unit; this is
NOT an F(ab')2 fragment. USe the RasMol command
restrict *l,*h to display only one F(ab') fragment.
Here is the crystal structure of a F(ab')
1igf
(559Kb)[Bbk|BNL|ExP|Waw|Hal]
fragment. There are two F(ab') fragments in the asymmetric unit; this is
NOT an F(ab')2 fragment. USe the RasMol command
restrict *l,*h to display only one F(ab') fragment.
|
Last updated 7th April '97