Human ICE-protease

Human ICE-protease
Members of the ICE family of cysteine proteases have been found to be responsible for many of the proteolytic events during apoptosis, a fundamental biological process that is required to maintain the integrity and homeostasis of multicellular organism. These proteases display an unusual substrate specificity for an Asp at the cleavage site. This newly emerging protease family has been called the Caspase family (Cysteinyl aspartate-specific protease).
Apoptotic cell suicide is a major form of cell death, characterized initially by a series of stereotypic morphological changes.These changes are the result of convergence of biochemical pathways on cellular substrates, in which a major element is activation of caspases. The overexpression of these proteins induces apoptosis. Several types of stimuli-induced apoptosis are inhibited by caspase-specific inhibitors, indicating that caspase proteases are common mediators of apoptosis. During induction of apoptosis, caspase proteases are sequentially activated and thereafter cleave several cellular proteins essential for cell growth. The substrates of this reaction are widely dispersed in the nucleus, cytoplasm and cytoskeleton. Caspase activation is the end result of physiological or pathological stimuli. Pathological stimuli include damage to cell membranes, mitochondrial function, other critical intracellular organelles and DNA. Several, distinct agents are known that may be part of the signaling pathways that couple injury to these cellular components to apoptosis: ceramide, collapse of mitochondrial transmembrane potential, p53 activation. Other stimuli are signaled through cytokine receptors (such as fas/APO-1/CD 95 and TNFRI and II) or transcription factors (such as p53, IRF-1 and rb). The transduction of these stimuli into caspase activation is regulated by a large family of proteins (the bcl-2 family).
ICE (caspase-1)
Interleukin-1ß-converting enzyme (ICE/caspase-1) was the first member of the caspase family to be identified as a novel type of cysteine protease responsible for the conversion of precursor interleukin-1 beta to its mature form in monocytes. The mature form of IL-1ß, cleaved at Asp-116-Ala-117 is a key mediator of inflammation. ICE was found to be a mammalian homologue of the Caenorhabitis elegans cell death protein Ced-3. This initiated studies about its possible role in programmed cell death. However, ICE knockout mice develop normally, with no apparent physiological or morphological aberrations, indicating that ICE has no substantial role in apoptosis. ICE defines a subfamily in the caspase family of cysteine proteases, two other family members (caspase-4, caspase-5) cluster around it in the phylogenic tree.
Caspase-2
Overexpression of the full caspase-2 mRNA in some cell types results in apoptosis, whereas overexpresson of an alternative caspase-2 splice variant suppresses apoptosis induced by serum withdrawal, suggesting that caspase-2 has a positive and a negative role in the regulation of the apoptotic process. Caspase-2 may be activated in vitro by caspase-1, caspase-3 and the neutral serine protease granzyme B which is stored in the specialized lytic granules of cytotoxic lymphocytes. Caspase-2 is expressed at high levels in the central nervous system, liver, kidney and lung during embryonic development. Caspase-2 belongs to the CED-3 subfamily (defined by C. elegans caspase).
Caspase-3
This protease is one of the key executioners of apoptosis. It is widely distributed, with high expression in cell lines of lymphocytic origin. Caspase-3 prefers substrates with a DXXD sequence. His substrates in apoptosis can be identified based on this recognition motif. Caspase-3 deficient mice die at 1-3 weeks of age with characteristic morphological aberrations. Neuronal apoptosis is markedly failed in these mice resulting in hyperplasias, ectopic cell masses and duplicated brain structures. Nevertheless other major organs are uneffected by the mutation. These results indicate that caspase-3 has a non-redundant role in neural apoptosis whereas in other tissues other isoforms can substitute for its loss. Caspase-3 is activated by granzyme B in vitro.
Caspase-4, Caspase-5
Caspase-4 and caspase-5 are the members of the caspase-1 subfamily.
The caspase-4 mRNA can be found in most tissues, with the exception of
brain. It can also be detected in the lung, liver, ovary and placenta,
where caspase-1 mRNA is barely detectable. Caspase-4 and caspase-5 have
different substrate specifities from that of caspase-1: pro-IL-1ß
is a poor substrate of these forms.
Caspase-4 may be involved in caspase-1 activation. The overexpression
of both caspase-4 and caspase-5 results in apoptosis.
Caspase-6
Caspase-6, a member of the CED-3 subfamily has two alternatively spliced
isoforms. The overexpression of the full length cDNA, but not the shorter
splice variant, results in apoptosis. Caspase-6 can activate pro-caspase-3
and conversly, the two proteases may form an amplification cycle.
Caspase-7
Caspase-7, a member of the CED-3 subfamily also has two splice isoforms, one of which can act as a negativ regulator of apoptosis. Caspase-7 is widely expressed in the body, with lowest expression levels in the brain. Granzyme B activates the inactive pro-form of the enzyme.
Caspase-8
Caspase-8 is probably at the apex of the apoptotic cascade. It
can activate all the other family members. Its activation is triggered
by its association with the DED domain of FADD, an adaptor protein, playing
a role in the assembly of a death-inducing signalling complex. The complex
is formed when CD95, a cell surface cytokin receptor is activated, and
binds FADD and caspase-8. Caspase-8 contains two N-terminal stretches homologous
to the DED domain of FADD, these may play a role in FADD association. The
overexpression of the protease induces apoptosis, but the coexpression
of some of its isoforms blocks cell death.
The active site has an unusual QACQG pentapeptide.
Caspase-9
Caspase-9 is a member of the CED-3 subfamily. Its active-site pentapeptide differs from that of the other family members being QACGG instead of QACRG. Caspase-9 has multiple mRNA forms.The proenzyme form is activated by caspase-3 and granzyme B in vitro.
Caspase-10
This enzyme, similarly to caspase-8 has a QACQG active site and contains two FADD-like DED stretches in the N-terminal domain. Its mRNA is present in most tissues. Recombinant caspase-10 processes all caspases, it may lie near the apex of the apoptotic cascade.
The human caspase sequences cluster into two subfamilies: the ICE and the CED-3 subfamily. The relationships are based on the full length proenzymes. The dendogram is not affected, if the prodomains are left out from the sequences.
Phylogenetic relationships in the
ICE family of cystein proteases. The proteins can be grouped into two subfamilies,
based on their sequence homologies to ICE (caspase-1) and CED-3 (the C.
elegans caspase).
All the caspases contain a conserved QACXG (where X is R, Q or G) pentapeptide active-site motif.The active-sites and the alternative names of the caspases are shown in this table.
Caspases are synthesized as inactive proenzymes with an N-terminal prodomain and two subunits sometimes separated by a linker peptid. During maturation the prodomain and the linker peptide are cleaved at specific Asp residues, resulting in the active caspase of two subunits.Family members are sequentially activated, they form a proteolytic cascade.
The mature form of interleukin-1ß-converting enzyme, the best characterised member of the ICE family is derived from a precursor of 404 amino acids which undergoes proteolysis. The N-terminal 119 amino acids and an integral fragment spanning residues 298-316 are removed. The active form thus comprises a p20 (residues 120-297) and a p10 (residues 317-404) subunit, both of which are essential for activity.
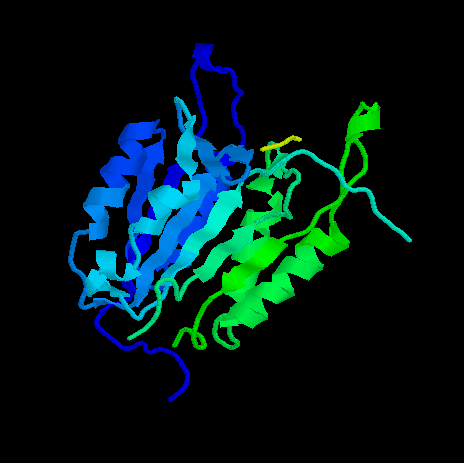
 The
p20 and
p10 subunit of
the active ICE heterodimer
The
p20 and
p10 subunit of
the active ICE heterodimer
The cleavage sites of the maturation process have been determined in
most caspases and are summerized in the table below.
| CASPASE | CLEAVAGE SITE |
| Caspase-1 | Asp-103, Asp119, Asp-297, Asp-316 |
| Caspase-2 | Asp-152 (exact site not known), Asp-316, Asp-330 (cleavage sites are based on equivalent sites in caspase-2) |
| Caspase-3 | Asp-9/Asp-28, Asp-175 |
| Caspase-4 | Asp-104, Asp-270, Asp-289 |
| Caspase-5 | Asp-121 (exact site not known), Asp-311, Asp-330 |
| Caspase-6 | Asp-23, Asp-179, Asp-193 |
| Caspase-7 | Asp-23, Asp-198, |
| Caspase-8 | Asp-210, Asp-216, Asp-374, Asp-384 |
| Caspase-9 | Asp-130 (exact site not known), Asp-315 (cleavage by granzyme B)/ Asp-330 (cleavage by caspase-3) |
| Caspase-10 | Asp-219, Asp-372 |
Here is a site to examine the secondary structure of ICE.
The structure of caspase-1 and caspase-3 has been determined by X-ray diffraction. The overall structures are very similar, but major differences can be seen around the active site, especially in the substrate-binding pocket. The crystal structures of both caspase-1 and caspase-3 show that the active enzyme is a tetramer, containing two small and two large subunits. A small and a lagre subunit form a heterodimer, two of these heterodimers form the tetramer (or homodimer of the two heterodimers).
The structure of ICE/caspase-1 has been solved at 2.5A resolution. The subunits of the heterodimer derive from an inactive 45K proenzyme. The proteolytic processing leads to a 20K and a 10K (p20 and p10) subunit, both of which are necessary for full proteolytic activity. The protease was crystallized in the mature form in complex with a tetrapeptide inhibitor.
TOPOLOGY OF THE HETERODIMER
The p20 p10 heterodimer appears as a single domain. This comprises the crystallographic unique molecule. The molecule consists of a single domain, it is an alpha/beta protein. The enzyme core is a six-stranded beta sheet with five parallel strands (residues 164-170, 199-205, 230-236, 278-283, 327-331) and one anti-parallel strand (residues 388-393). Six alpha helices (residues 139-149, 182-196, 207-226, 257-269, 348-363, 366-377) lie around the beta sheet core, the helices run roughly parallel to the beta strands.
The beta sheet core is shown in this picture.
The alpha helices flanking the beta sheet are shown red in the next picture. The beta sheet viewed from this side shows the characteristic left-handed twist typical of a predominantly parallel beta sheet. The twist measures 60o.
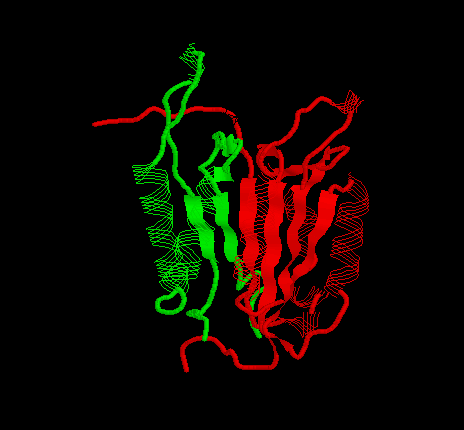
In the beta sheet the first four strands are contributed by p20 and all lie parallel to each other. The remaining two strands are from p10, the first runs parallel to the p20 strands, the final one runs anti-parallel to the rest. The ordering of the beta strands in the sheet is 3,2,4,5,8,10, following the nomenclature in the polypeptide chain. Beta strands 1, 6, 7, 9 and 11 does not take part in the formation of the beta strand.
The beta strand is shown in the next picture. The four strands derived from p20 are coloured blue, the two strands from p10 are green.

In the ICE structure three beta-alpha-beta motifs can be identified together with a beta-alpha-alpha-beta unit. The beta-alpha-beta units are all in p20, they are all left-handed, formed from sheet2-helix2-sheet3, sheet2-helix3-sheet4, sheet4-helix4-sheet5, as shown in the next picture.
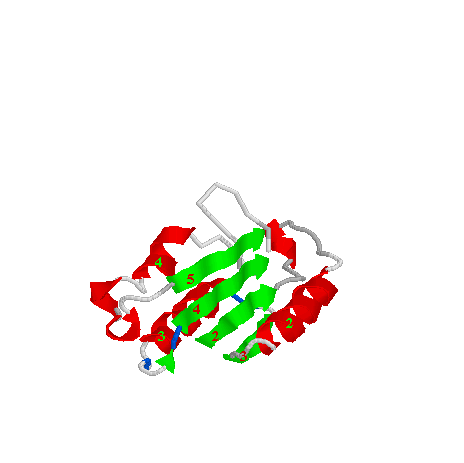
The beta-alpha-alpha-beta motif is formed in p10 from helices 5 and 6 and strands 8 and 10. This can be seen here:

Here is a complete description of the secondary structural elements
in
p20 and in p10.
The catalytic machinery involves a diad composed of a cystein sulphydryl group (Cys285) and a histidine imidazol ring (His237). Both of them are on the p20 subunit.The tetrahedral transition state of the cystein protease is stabilised through hydrogen-bonding with the backbone amide protons of Cys285 and Gly238.
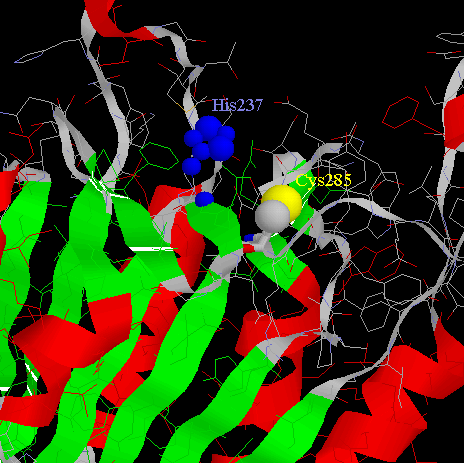
The P1 Asp of the substrate seems to be stabilized by four
residues: Arg179, Gln283 from p20 and Arg341, Ser347 from p10.
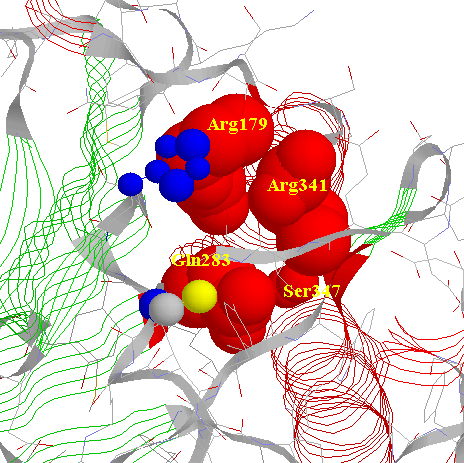
Here you can see a schematic drawing of the interactions in the active site with a tetrapeptide inhibitor.
A Rasmol visualisation looks like here:
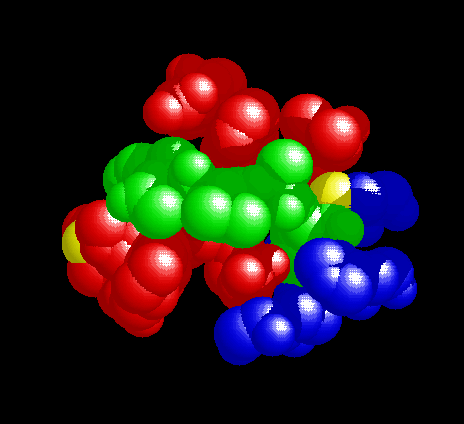
The distinct specificities of caspase-1 and caspase-3 arise from the important differences in the S4 subsites (the site accomodating the P4 residue of the substrate) of the enzymes.The large hydrophobic depression in caspase-1 easily accomodates a tyrosyl sidechain, present in the P4 position of pro-IL-1ß and the tetrapeptide inhibitor.
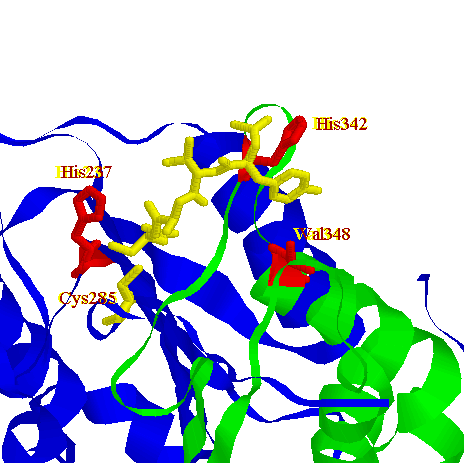
In caspase-3 there is a surface loop spanning residues Phe380-Phe389 which does not have a counterpart in caspase-1. In caspase-3 the S4 site is significantly smaller, capable of binding the carboxylated sidechain of P4 Asp. This geometry explains the enzymes strict requirement for an Asp in the P4 position of substrates and inhibitors.
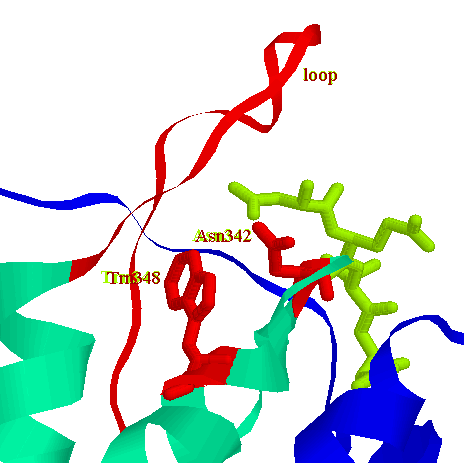
Several of the sites responsible for distinct specificities of caspase-1
and caspase-3 are conserved in other family members, suggesting a similar
substrate preference for these enzymes.
The ICE protease crystallized in tetramer form. The two p20-10 heterodimers formed a tetramer, or homodimer of heterodimers. The 2-fold axis runs approximately at the center of the sixth, antiparallel beta strand (beta 10). The beta strand in the other dimer hydrogen bonds to this strand, thus the two beta sheets form a sheet of 12 beta strands, in which beta strand 10 and 10' run anti-parallel to one other. The left-handed twist in the core beta strand becomes 125o. In the tetramer the C-alpha atoms of Asp297 in the p20 and the p10' Ala317' are just 5.3A apart. The terminal beta strands (beta 6-beta 7' and beta 6'-beta 7) run antiparallel with six hydrogen bonding interactions. The distance between the p20 C-terminus and the p10' N-terminus suggests that in the active enzyme p20 and p10' (and conversely p20' and p10) derive from the same polypeptide chain. An alternative model suggests that the heterodimers are formed from one precursor and the active tetramer results from the association of two heterodimer.
The central role of the members of the caspase family in apoptosis has
been substantiated by a number of studies. Many things are known about
the enzymology of these proteases, their activation pathways, substrates
and inhibitors, the crystal structure of two forms has been determined.
The understanding of the regulation and structural-functional relationships
in this protease family is very important, since inappropriate apoptosis
underlies many of the human diseases including cancer and neurodegeneration.
Despite these major advances this field remains a fertile ground for further
studies on these enzymes and the physiological processes they are involved
in.
Gáspár Jékely
jekely@enzim.hu
PhD student
Hungarian Academy of Sciences
Institute of Enzymology
Neuroenzymology Group
Karolina út 29.
1113 Budapest
Hungary