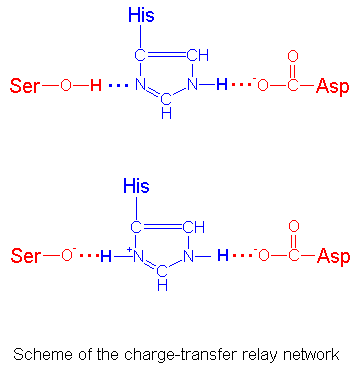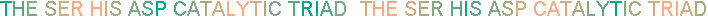
Serine proteases have independently evolved a similar catalytic device
characterized by the Ser, His, Asp triad (Ser-195, His-57, Asp-102 in
chymotrypsin, trypsin and elastase; Ser-221, His-64, Asp-32 in the
bacterial subtilisin).
The serine in the triad is much more reactive then other serines in
the protein. The serine hydroksyl is normally protonated at neutral pH, but
in the enzyme Ser-195 (Ser-221) is hydrogen-bonded to His-57 (His-64),
which is further hydrogen-bonded to Asp-102 (Asp-32). These three amino
acids are often referred to as a catalytic triad.
As the serine oxygen attacks the carbonyl carbon of a peptide bond,
the hydrogen-bonded His functions as a general base to abstract
the serine proton, and the negatively charged Asp stabilizes the positive charge
that forms on the His residue. This prevents the development of a very
unstable positive charge on the serine hydroxyl and increase its nucleophilicity. The residues of the catalytic triad form a charge-transfer relay network.