There are many potential glycosylation sites in a protein, but only about one third of them is utilized. For example the rapid folding of a fragment of protein containing the glycosylation site can prevent the transfer of oligosaccharide chain. Thus the conformation of the protein is very important. It is known that glycosylation sites within beta turns are favoured. The degree of glycosylation also depends on oligosaccharyltransferase activity and the availability of completely glycosylated precursor.
The polypeptide chains of glycoproteins are synthesized under genetic control. The carbohydrate chains are enzymatically generated and covalently linked to the polypeptides. The quantities of available processing enzymes are generally not sufficient to ensure the synthesis of uniform products. This results in microheterogenity - the glycoproteins have variable carbohydrate compositions.
The glycosylation reactions occur in the lumen of Endoplasmic Reticulum (ER) and in the lumina of the cis-, medial- and trans-Golgi vesicles.
There is the difference in biosynthesis of N- and O-linked oligosaccharides. O-linked carbohydrates are added one at time and each sugar transfer is catalyzed by a different enzyme. (See Biosynthesis of O-glycans ). In contrast, during forming of N-glycans, a large oligosaccharide chain containing 14 sugar residues is added to the Asn residue. It occurs in the ER and then, in the Golgi some sugar residues can be removed and the others can be added (See Biosynthesis of N-glycans )
Monosaccharides are joined together and to protein by the glycosidic bonds. Formation of these bonds requires free energy input which is acquired through the conversion of monosaccharide units to nucleotide diphosphate or monophosphate sugars (Enzymes using nucleotide sugars as glycosyl donors during biosynthesis of oligosaccharides belong to Leloir pathway enzymes ; the non-Leloir pathway enzymes use glycosyl phosphates).
The ester bond between the phosphate group and the carbon atom in the sugar is a high-energy bond.Thus the transfer of the sugar residue to a hydroxyl group of another sugar or amino acid residue (or to amide group of Asn) is energetically favoured.
This process is performed by glycosyltransferases. They are integral membrane proteins with active sites in the lumen of organelle.
NUCLEOTIDE SUGARS USED BY THE GLYCOSYLTRANSFERASES DURING GLYCOSYLATION
* UDP : N-Acetylglucosamine (GlcNAc) ; N-Acetylgalactosamine (GalNAc) ; Galactose (Gal); Glucose (Glc); N-Acetylmuramic acid (NAM) ; glucuronic acid ; xylose
* GDP : Fucose ; Mannose
* CMP : Sialic acid (Neu-5Ac)
Nucleotidesugars are synthesized in the cytosol via following reaction:
sugar-1-phosphate + XTP ----------------- sugar-XDP + PPi
This process is catalyzed by nucleoside transferase and pyrophosphorylase.
An exception is CMP-Neu-5Ac which is synthesized from the sugar ( not sugar phosphate) and CTP :
Neu-5-Ac + CTP --------------------CMP-Neu-5Ac + PPi
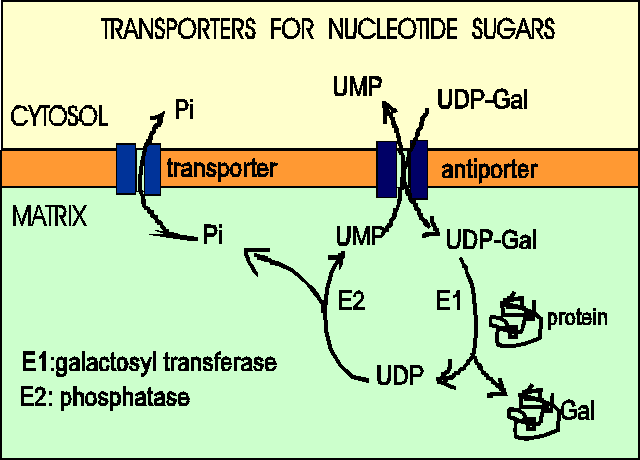
The ER and Golgi membranes contain transporters for nucleotide sugars (they are antiporters : when sugar nucleotides are imported, nucleotides are exported fromthe ER or Golgi vesicles.
Go to :
Biosynthesis of O-linked oligosaccharides
Biosynthesis of N-linked oligosaccharides
Biosynthesis of GPI membrane anchors