TABLE OF CONTENTS
Free radicals in human body can arise from fatty food, smoking, alcohol, environmental pollutants, hydrogen peroxide, pollutants, ozone, toxins, carcinogen toxins, ionisation etc. The vast majority of free radicals come from within the body, an unavoidable by-product of living system. Free radical intermediates are produced in living systems under normal conditions, the body handles free radicals formed by the breakdown of compounds through the process of metabolism. The major sources of free radicals (such as O2- and HO2·) are modest leakages from the electron transport chains of mitochondria, chloroplasts and endoplasmic reticulum.
The resulting free radicals, such as superoxide anion (O2-) and hydroxyl radical (OH·), as well as the non-radical hydrogen peroxide, can damage macromolecules, including DNA, proteins and lipids. Likewise, other products of oxygen metabolism, such as hypochlorous acid, chloramines, and oxidised lipids have all been related in such damages. The superoxide radical, although it is unreactive in comparison with many other radicals, biological systems can convert it into other more reactive species, such as peroxyl (ROO·), alkoxyl (RO·) and hydroxyl (HO·) radicals.
There are four types of free radicals damages:
Fortunately, the body also has several natural chemical means or systems for neutralizing free radicals. There is agents that counteract and minimize free radical damage and their function is to donate or provide unpaired electrons to which free radicals can attach without causing harm. Such "cell-savers" are called "Antioxidants."
Antioxidants get their name because they combat oxidation. Oxidation is a reaction in which a molecule looses an electron. The two major sources of antioxidants are:
And there are some types of antioxidants, like the Flavonoids, found in the skin and seeds of fruits, possess the ability to physically capture free radicals until these are actually removed from the body. Others, like Sulphorophane, found in broccoli, tend to enhance the body’s own free radical scavenging mechanism. And finally, the ones like L. Limonene, phytochemical found in citrus fruit peels, can actually perform both actions. Some popular antioxidants today include Vitamin E, Vitamin C, Vitamin A, which can be taken under a health supplement form or through fruits, vegetables, fish oil, green tea, sesame oil, and Genistein from soy bean shown to be cancer preventive. Some antioxidants come from minerals, such as selenium, copper, zinc (they are considered antioxidants because they work together in conjunction with an antioxidant enzyme and are necessary for the enzyme to function properly).. etc. The list of dietary antioxidants goes on and on, and scientist are continually discovering more.
Often, free radical reaction involve, either directly or indirectly, the formation of oxyradicals. Molecular oxygen is, in fact, a biradical possessing two unpaired electrons of parallel spin (Fig.1). This electrons exist at their lowest energy level when unpaired and when they have spins in the same direction. This configuration is called the ground or triplet state and it describes the paramagnetic and electronic behaviour of oxygen.
Most molecules have electrons with opposite spins and are termed diamagnetic, their ground states are described as singlets. Two forms of singlet oxygen exist (Fig.1).
The transfer of electrons to oxygen can also lead to the production of toxic free radical species. The best documented of these is the superoxide radical, shown in Fig.1. A two electron reduction of oxygen results in the formation of the peroxide ion (Fig.1). This is not a free radical, but is very reactive and at physiological pH protonates to form hydrogen peroxyde (H2O2). The reaction of hydrogen peroxyde with superoxide radical can lead to a series of reactions wich produce additional reactive species. These are described below.
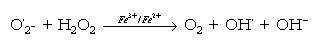

Free radicals often reacts with other molecules in a manner that initiates the formation of many more free radical species. It is this self-propagating ability that makes them so toxic to living organisms. Thus, free radicals are generated by several mechanisms:
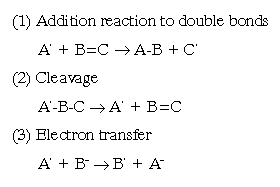
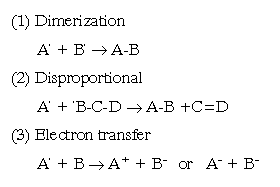
A single initiation event which lead to the production of one free radical can soon produce many more; their is called "cascade", and it can be prevented via the termination steps above. In cells, termination also occurs by the interaction of protective mechanism and antioxidants.
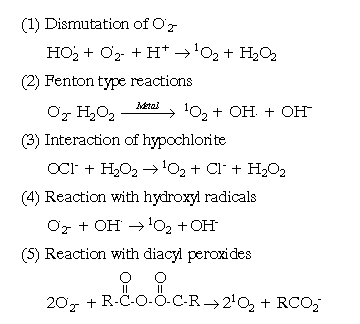
Photosensitised reactions are very important too, because they are involved in production of cellular (1O2) . A photosensitive molecule is one which on activation by radiation or light causes another molecular component to react. Two types of photoreactions exist:
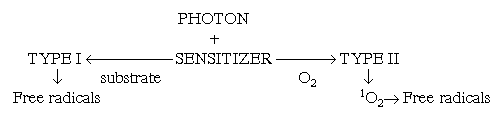
The flux along these pathways is very much dependent on the concentrations of the substrate and molecular oxygen. If the concentration of oxygen is high but the substrate concentration is low, the Type II reactions is favoured. Type I reaction are more efficient if the reverse conditions are prevalent. The fate of the excited species in both types of reaction is most important, particularly as more excited species and free radicals can be produced. Sensitises can also undergo electron transfer reactions with the substrate or oxygen:
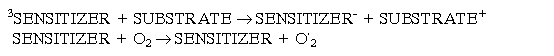
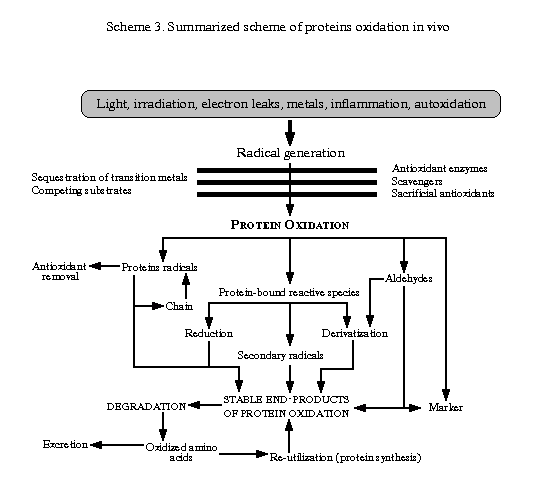
Formed (1O2) and various types of free radicals can react with many different organic molecules especially lipids. As a consequence, membranes are major targets of attack. The nucleic acids (particularly guanine), proteins and enzymes, are also susceptible. And in next section we will try to describe free radicals damages to proteins.
Radical-mediated protein oxidation has been studied throughout the century. In the first decade , Dakin published detailed chemical studies of the oxidation of leucine and other amino acids by Fenton systems (transition-metal ion plus hydrogen peroxide), and protein agregation and fragmentation was detected by others. Soon after the discovery of gluthatione, Hopkins appreciated that this reductant could be both an anti- and pre-oxidant and inactivate proteins. Several authors assessed the proteolytic susceptibility of oxidized proteins, and demonstrated biphasic effects, whereby limited oxidation leads to enhanced susceptibility, while more extensive oxidation may be associated with increasing resistance.
In many works there was detected that amino acids in many various reactions can form carbonyls, such as the oxo acids and aldehydes with the same or less carbon atom then the parent amino acid; e.g. glycine giving rise to glyoxsal and glyoxylic acid, formaldehyde and formic acid; alanine giving rise to acetaldehyde and acetic acid, etc. This general scheme has been confirmed for many amino acids, including aromatic amino acids, although other reactions may also occur.
During the oxidation of aliphatic amino acids by HO·, hydroxilated derivates, notably of the side chains, are formed. During the oxidation of aromatic residues, the formation of phenoxyl radicals from tyrosine, and their conversion into dityrosine and further products, can occur, especially if there are no reductants to repair the tyrosil radicals (e.g. thiols, vitamin E) and if there are vicinal tyrosil radicals. Hydroxylation of phenylalanine, tyrosine and tryptophan is also a characteristic reaction of hydroxyl radicals, and similar reactions of histidine (giving 2-oxohistidine) are important. Histidine in reactions with free radicals can form some imidazole decay products or in some cases aspartic acid and can form some histidine derivates (Fig.2.). Fenton chemistry can generate both the aliphatic and aromatic products.
Early radiation studies on lysozyme, ribonuclease and other enzymes were carried out mainly in the absence of O2 and showed that HO· was the most effective inactivator, and characterised other more selective (but less efficiently inactivating) species such as (SCN)2-·, Br2-·, Cl2-· and I2-·. For example, (SCN)2-· was found to react with an important tryptophan residue in pepsin and so inactivate the enzyme, although damage could be reversed.
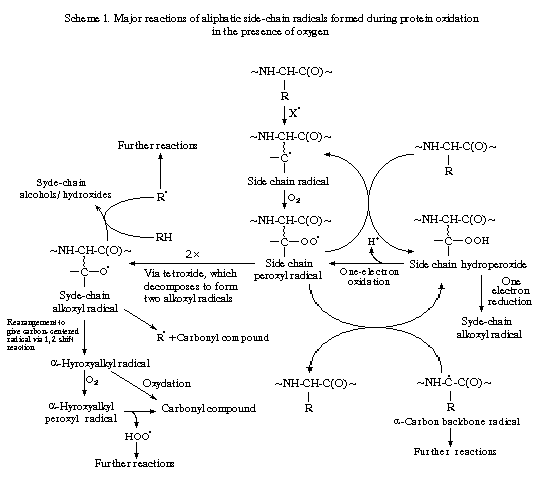
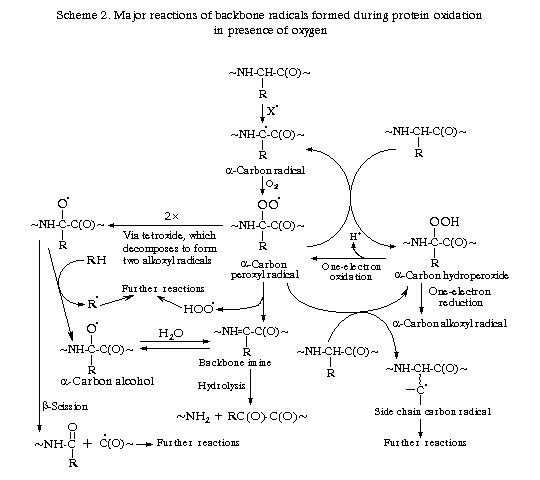
Table 1. summarises some products of protein oxidation. Schemes 1 and 2 illustrate some of the major reactions believed to be important in the oxidation of the side chain and the backbone respectively. Alkoxyl radicals apparently have a greater importance in protein oxidation chains than they do in lipid peroxidation, in which peroxyl radicals are key chain propagating species.
| TABLE 1. Some product of protein oxidation |
| Tyr+HO· or RNIs (reactive nitrogen species) | Dopa |
| Tyr+HOCl | 3-Chlortyrosine |
| Tyr+RNIs | 3-Nytrotyrosine |
| Tyr+HO·, or one electron oxidation of Tyr or HOCl, followed by radical- radical combination | Dytirosine |
| Phe+ HO·, one electron oxidation | o- and m-tyrosine |
| Phe+ HO·, before or after dimerization | Dimers of hydroxylated amino acids |
| Trp+ HO·,or on electron oxidation | N-Formylkynurenine; kynurenine |
| Trp+ HO·,or on electron oxidation | 5-Hydroxytryptofan; 7-hydroxytryptofan |
| His+ HO·, or one electron oxidation | 2-Oxohistidine |
| Glu+ HO· in presence of O2 | Glutamic acid hydroperoxide |
| Leu+ HO· in presence of O2 | Leucine hydroperoxides and hydroxides; a-ketoisocapronic acid; isovaleric acid; isovaleraldehyde; isovaleraldehyde oxime; carbonyl compounds; |
| Val+ HO· in presence of O2 | Valine hydroperoxides and hydroxydes; carbonyl compounds; |
| Lys+ HO· in presence of O2 | Lysine hydroperoxides and hydroxydes; carbonyl compounds; |
| Pro+ HO· in presence of O2 | Proline hydroperoxides and hydroxydes; 5-hydroxy-2-aminovaleric acid; carbonyl compounds; |
| Arg+ HO· in presence of O2 | 5-Hydroxy-2-aminovaleric acid; |
| Ile+ HO· in presence of O2 | Isoleucine hydroperoxides; isoleucine hydroxides; carbonyl compounds; |
| Gly: hydrogen atom abstraction from a-carbon followed by reaction with CO2·- radicals; | Aminomalonic acid |
| Met+ HO· or one electron oxidation | Methionine sulphoxide |
| Cys+ HO· or other hydrogen atom abstracting species | Cystine; oxy acids |
| All amino acids exposed to photo-oxidation, oxidizing radicals or HOCl | RCHO species formed by decarboxylation or deamination |