II. Biosynthesis
Beginning with GlcNAc the whole pentasaccharide core is synthesized first linked to dolichol pyrophosphate (Dol-P) and independent of the protein that
will be glycosylated in a later step.
The biosynthesis of the oligosaccharide precursor molecule starts at the cytosolic site of the rough ER (RER) with the attachment of GlcNAc from UDP-GlcNAc to
Dol-P. This step can be inhibited by tunicamycin. Subsequently the rest of the core sugars become added to the molecule step by step to yield Manp5-GlcNAcp2.
This structure stays then attached to P-P-Dol but is transferred to the lumen site of the RER where additional
processing occurs before the actual glycosylation of the protein takes place cotranslationally. The whole process is shown in the following picture.
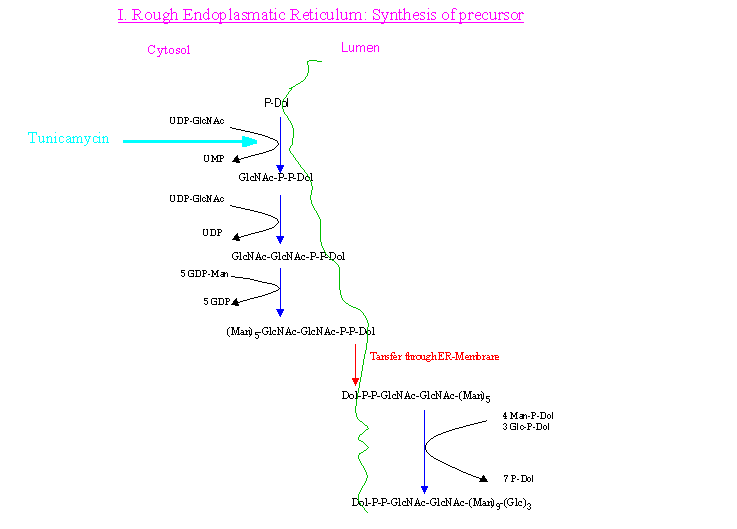
In general, the degree of glycosylation is dependent on
- the available amount of completely glucosylated precursors,
- oligosaccharyltransferase activity (for more detailed information on the enzyme itself see Kaplan (1988)), and
- the number of available Asn-X-Thr/Ser sites in the protein and their conformational accessibility.
After the the precursor molecule Glcp3-Manp9-GlcNAcp2 has been assembled , its transfer
to Asn by oligosaccharyltransferase (inhibitors are tunicamycin and amphomycin) is followed by further processing by two membrane-bound glucosidases which remove the terminal Glc residues. These glucosidases can be inhibited by 1-deoxynojirimycin and N-methyl-1-deoxynojirimycin.
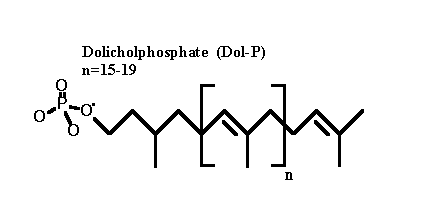
The released dolicholpyrophosphate is hydrolysed by a phosphatase to yield dolicholphosphate (Dol-P). (Dol-P cycle) This phosphatase can be inhibited by bacitracin.
In a first step the terminal Glc is removed by the action of alpha-1,2-glucosidase I. The remaining two residues are then cleaved by
alpha-1,3-glucosidase II. It is assumed that the three Glc residues function as a signal that prevents degradation of the oligosaccharide prior to
its transfer onto the protein.
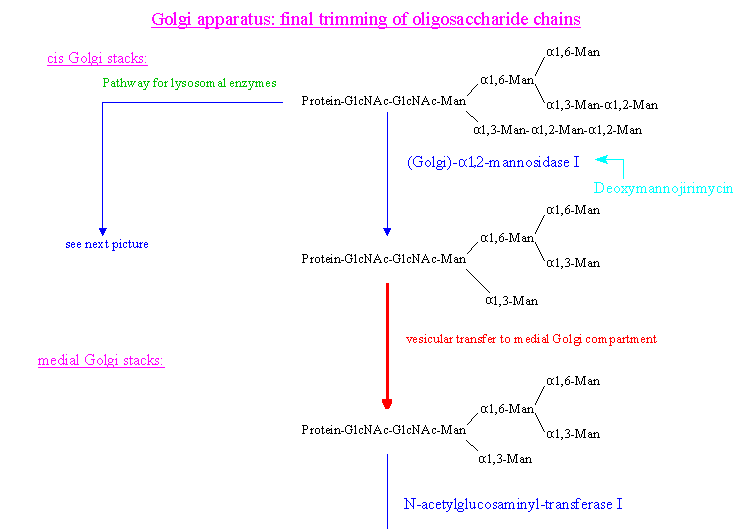
After the Glc residues have been removed, one Man residue is cleaved from the Man(alpha-1,6) branch by ER alpha-1,2-mannosidase.
The glycoprotein with the linked Manp8-GlcNAcp2 (high mannose type) side chain is then transported from the RER to the cis cisternae
of the Golgi stacks. This transport is thought to be receptor mediated with the receptor recognizing the deglucosylated oligosaccharide chain and can be inhibited with
Brefeldin A. (For a recent review article about the glycosidases (glucosidases and mannosidases) in these first steps of oligosaccharide trimming see Moremen 1994.)
RER: Transfer of precursor and subsequent trimming.
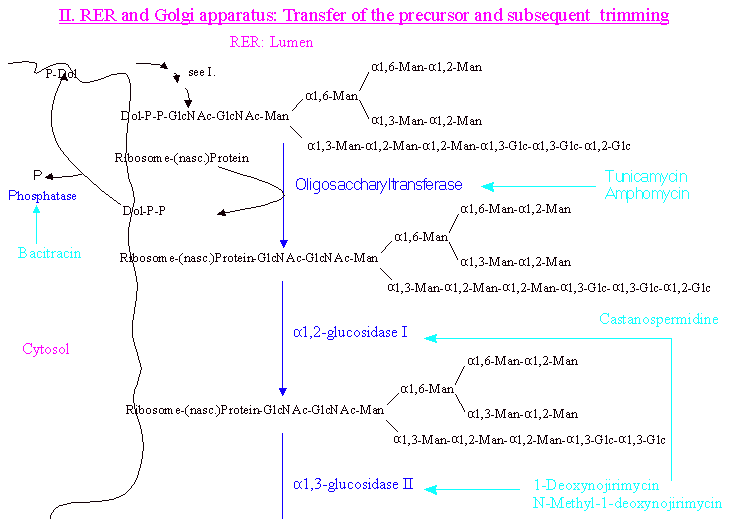
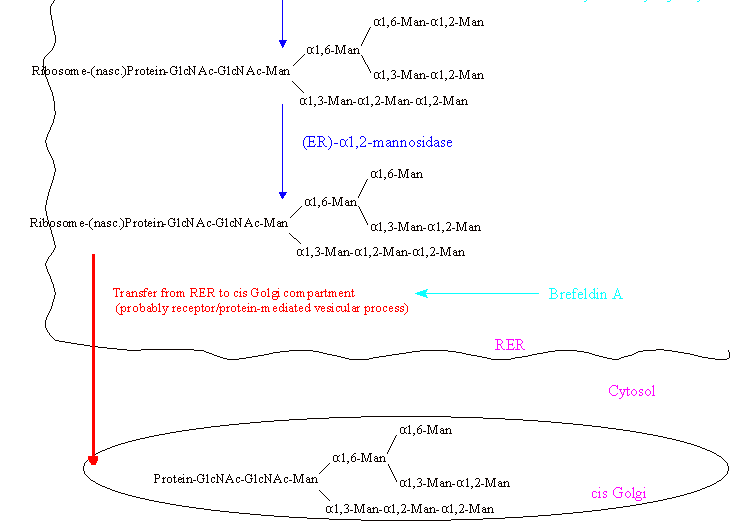
In the Golgi stacks further processing occurs depending on the final destination of the glycoprotein, which is solely determined by its
amino acid sequence. Currently two processing pathways have been investigated separating
- lysosomal enzymes (1) (which presumably contain at least one domain of conserved amino acid residues, e.g. Lys residues, (Baranski 1990)) which serves a binding site for the phosphotransferase)
from
- nonlysosomal enzymes (2)
(1) Two Man residues (of high mannose type sidechains) of the lysosomal enzymes become C-6 phosphorylated in a two step process in the cis Golgi cisternae:
1. Glucophosphorylation by N-acetylglucosaminylphosphotransferase (lack of this enzyme is the cause the disease mucolipidose II (I-cell disease, because of the
inclusion bodies found in all types of cells in all organs of the body))
2. Removal of Glc by action of N-acetylglucosamine-1-phosphodiester alpha-N-acetylglucosaminidase
These Man-6-P residues then interact with the corresponding receptor for the transport to the lysosomes.
(2) Non-lyosomal enzymes undergo the second pathway where Man residues are successively cleaved from the high mannose type chain to
yield precursors for the synthesis of complex and hybrid type oligosaccharide side chains.
In this pathway the following enzymes, distributed over the cis, medial and trans Golgi cisternae (see pictures), are involved:
- Golgi mannosidase I (alpha-1,2 specific): cleaves 2 Man residues from the Man(alpha1,3) branch to yield Manp
5-GlcNAc2
- N-acetylglucosaminyltransferase I : links a GlcNAc residue beta-1,2 to the terminal Man residue of the Man(alpha1,3) branch
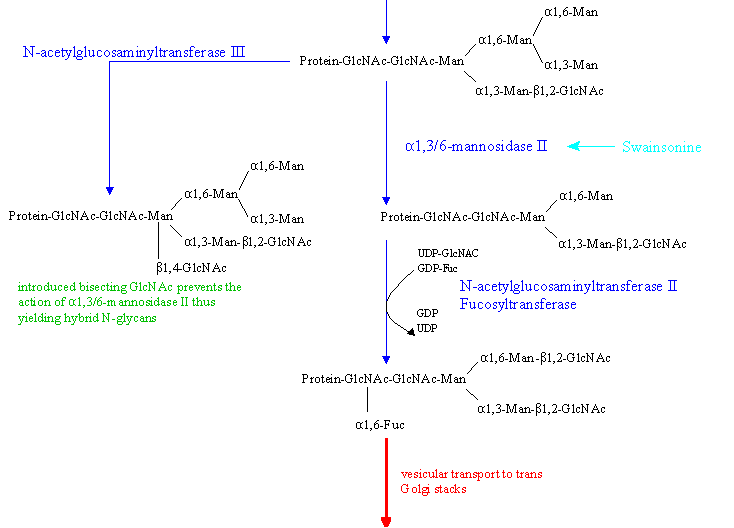
- Golgi alpha-mannosidase II : cleaves 2 Man residues from the Man(alpha1,6) branch leading to GlcNAcp-Manp
3-GlcNAc2
(can be inhibited by swainsonine, leading to abolishment of the synthesis of complex N-glycans)
If a bisecting GlcNAc residue is introduced by N-acetylglucosaminyltransferase III, prior to the action of mannosidase II the enzyme cannot remove the 2 Man residues mentioned above. This results in
the formation of hybrid type oligosaccharide sidechains.
Following the action of the mannosidase
- N-acetylglucosaminyltransferase II adds a GlcNAc residue beta-1,2 at the (now) free Man(alpha1,6) arm yielding the precursor for biantennary complex type structures.
Mainly in plant cells, but also in some mammals, might now occur the addition (alpha1,6) of Fuc to the GlcNAc residue at the 'reducing' end of the carbohydrate chain by action
of a fucosyltranferase.
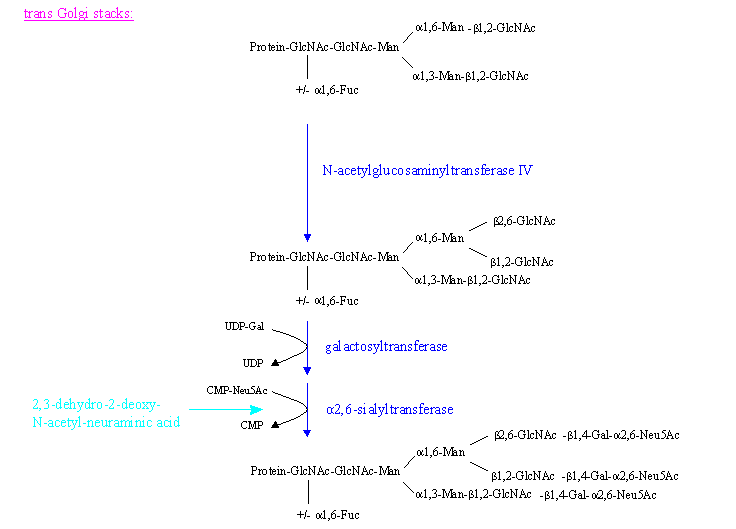
- Galactosyltransferase / Sialyltransferase: finally Gal residues might be linked to the terminal GlcNAc molecules (mainly in beta-1,4 manner but also alpha/beta-1,3
linkages have been found, but with the beta linkage occuring only on triantennary structures) followed by the addition of
sialic acid residues as terminators (end parts), which are thought to protect the glycoprotein carbohydrate chain and the protein from degradation.
Sialic acid is linked either alpha2,6 or alpha 2,3 to the preceding Gal. But there are also cases known where sialic acid becomes linked alpha2,8/9
to another preceeding sialic acid, yielding large clusters of sialic acid residues with for example up to 55 Neu5Ac residues, linked alpha2,8,found in N-CAM.These
sialic acid polymers have been predominantly found in neuronal cells. As has been shown by Paulson et al., that if alpha-2,3 sialylation takes place, it occurs prior to alpha-2,6 sialylation.
- Fucosyltransferase: together with the process of galactosylation and sialylation, a fucosylation in alpha-1,3 position of a GlcNAc residue at the nonreducing end might occur.
But it has been shown, so far, that either fucosylation or sialylation (in alpha2,6 linkage) occurs at the same branch not both (Paulson 1978; Beyer 1979)
(For a general model for the composition of glycosyltransferases see Paulson 1989 and Paulson 1987.)
During this process of further modifying the oligosaccharide chains the glycoprotein moves trough the Golgi apparatus from
the cis compartment through the medial cisternae and gets to its final place of modification of conformation and configuration in the trans Golgi cisternae before
it is then excreted.
- Higher branched complex type oligosaccharides are synthesized by the subsequent addition of further GlcNAc molecules by the corresponding
transferases. These can be added in a beta-1,4 linkage to Man(alpha1,3) or in a beta-1,6 linkage to Man(alpha1,6), or both to yield
2,4-; 2,6-tri and tetraantennary structures, respectively.
The type of synthesized complex type chain is determined by the order of action of the different GlcNAc transferases, as is true for the
action of galactosyl- and sialyltransferase.
- hybrid type instead of complex type carbohydrate side chains are synthesized because of the action of N-acetylglucosaminyltransferase III:
This enzyme introduces a 'bisecting' GlcNAc residue at the beta-1,4-linked core Man residue which subsequently prevents the action
of Golgi alpha-mannosidase II, therefore leaving the alpha-1,6 and alpha-1,3 Man residues attached to the Man(alpha1,6) branch. From
this GlcNAcp-Manp5-GlcNacp2 structure no further complex type directed degradation of the Man motif can occur. (Schachter 1989)
- high mannose chains are (mainly) not fucosylated due to the lack of GlcNAc linked beta-1,2 to the
Man(alpha1,3) branch, which is the required motif for the action of alpha-1,6 fucosyltransferase.
Comparable to this is the action of sialyltransferase and fucosyltransferase on the outer parts (Man(alpha1,3) of complex type chains:
Once Fuc has been linked to C-3 of GlcNAc, no alpha2,6-sialylation of the terminal Gal residue on the same branch occurs and vice versa.
But the fucosylated and sialylated (Sia linked alpha2,3 (!)) structure (Neu5Ac-alpha2,3-Gal-beta1,4-(Fuc-alpha1,3)-GlcNAc) occurs rather
often and is called Sialyl-Lewis X (SiaLeX). Here, the Fuc residue becomes attached to the GlcNAc residue after the addition of sialic acid.
The SiaLeX motif has mainly been found at the end of poly-N-acetyllactosamine chains.
- Polylactosamine oligosaccharide chains, which occur mainly on tri- and tetraantennary structures and preferably on the Man-alpha1,6-Man-beta1,4 branch, are presumably
formed by the successive action of beta-1,4 galactosyl- and beta-1,3 N-acetylglucosaminyltransferase (the key enzyme of the formation process) to yield:
[Gal(beta1,4)-GlcNAc(beta1,3)-]n (with n less than 50)as outer part of the pentasaccharide core structure.
Finally a sialic acid residue can also be added to the end of the polymeric structure.
In general, it can be said that the overall synthesis process is controlled by the amount and order/time of action of a specific enzyme (more than 150
glycosyltranferases have been found so far) in the respective Golgi compartment. (Paulson 1989)
In addition to these different types of carbohydrate chains further modification of the completely assembled structures might occur,
like phosphorylation and sulfation. Another special case is sialic acid which might be O-acetylated or might have a N-glycolyl group instead
of a N-acetyl moiety. The O-acetylation at C-4 is known to prevent the action of certain sialidases of bacteria and viruses.
Structure
General information about structures occuring besides the commonly found oligosaccharides and posttranslational modifications.
References
VSNS-PPS course
Written by: Christian Frosch
frosch@mzdmza.zdv.uni-mainz.de
http://www.uni-mainz.de/~frosc000
Institute of Toxicology
University of Mainz
Last update: 10.07.1995






