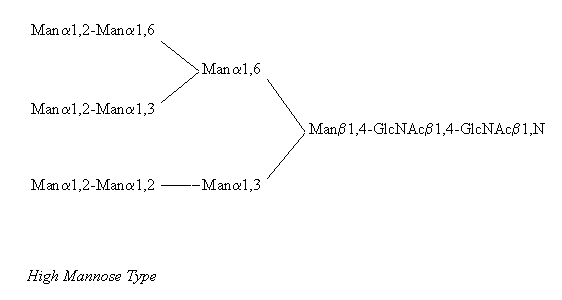
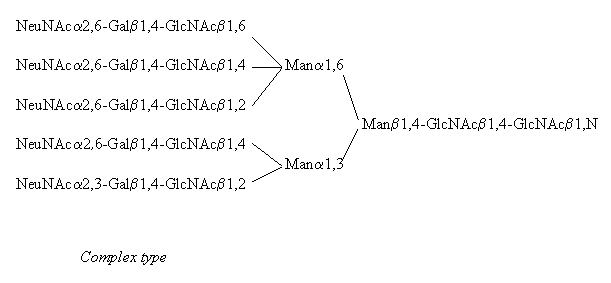
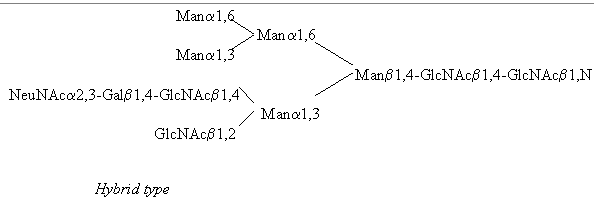
All N-linked oligosaccharides are linked to the amide N in the sidechain of Asn in the consensus sequence Asn-Xaa-Ser/Thr, where Xaa can be any amino acid besides Pro and Asp. N-glycans can be subdivided into three distinct groups called 'high mannose type', 'hybrid type', and 'complex type', with the common pentasaccharide core - Manp(alpha1,6)-(Manp(alpha1,3))-Manp(beta1,4)-GlcpNAc(beta1,4)-GlcpNAc(beta1,N)-Asn - occuring in all three groups. (Besides GlcNAc the following sugars have been found N-linked to Asn, mainly on bacterial glycoproteins: Glc, GalNAc and L-Rha, (Lis, Sharon 1993) but these will not be dealt with here)
Examples for all three types are provided in the following pictures, with the core residues shown on the right hand side of each structure.



Although only a pentaantennary complex type structure is displayed above, the complex type chains occur also as mono-, bi-, tri (2,4- and 2,6-branched), and tetraantennary structures and the amount and type of sialylation differs.
The relationship between all three types can be ascribed to the fact that they originate from one precursor oligosaccharide which contains the described common
pentasaccharide core Man3-GlcNAc2, and some additional sugar residues and the non-reducing end, and is then processed enzymatically to yield these three types. (see
biosynthesis)
Since the hydroxyl group of Ser/Thr is thought to be involved in hydrogen bonding during the enzymatic attachment of the oligosaccharide precursor molecule to yield a favorable conformation for the action of the oligosaccharyltransferase (Kasturi 1995), it has been suggested for proline that the steric hindrance might be to large (Kornfeld 1985), preventing glycosylation at Pro containing sites. The negative influence of aspartic acid towards glycosylation can be ascribed to the negative charge on the side chain of this residue. In addition some cases have been reported where Ser/Thr is replaced by cysteine. While Ser replacement by Cys generally leads to decreased glycosylation, it has been shown recently (Kasturi 1995) that substitution by Thr at a given potential glycosylation site can lead to increased glycosylation. This is in accordance with the model of hydrogen bonding being an important factor during the attachment of the precursor molecule to the protein. Although there are usually many potential glycosylation sites in a protein it has been estimated that glycosylation occurs only at one third of them. Mostly at those sites where the surrounding amino acids allow the formation of a beta turn.
Go to Biosynthesis
Go to General information about structures occuring besides the commonly found oligosaccharides and posttranslational modifications.
frosch@mzdmza.zdv.uni-mainz.de
http://www.uni-mainz.de/~frosc000
Institute of Toxicology
University of Mainz
Last update: 10.07.1995