Click here for a diagram
of the dimer,
Click here for a diagram
of the dimer,
Back to Quaternary Structure Index
Examine the crystal structure of a single subunit of horse liver alcohol dehydrogenase (this is the apo- form, i.e. there is no NAD+ coenzyme).
 Click here for a diagram
of the dimer,
Click here for a diagram
of the dimer,
 and here for a space-filling
model, where the NAD+ molecules are visible.
and here for a space-filling
model, where the NAD+ molecules are visible.
Examine the crystal structure .
The domain involved in subunit-subunit interactions includes a six-stranded parallel beta sheet- this is a doubly wound alpha/beta fold . The strands of each subunit are in an antiparallel alignment with respect to each other, giving a twelve-stranded mixed beta sheet.
 This is indicated in this
diagram of a portion of the interface region. Notice
the position of the alpha helix sequentially adjacent to the beta strand making
the interfacial contact.
This is indicated in this
diagram of a portion of the interface region. Notice
the position of the alpha helix sequentially adjacent to the beta strand making
the interfacial contact.
In order to visualize how two monomers pack together to form a dimer, bear in mind the positions of the side chains of His-105 and Tyr-286. The face of the histidine of one subunit stacks against the tyrosine of the other, and vice-versa.
 This diagram illustrates
this packing.
This diagram illustrates
this packing.
The following diagram indicates the positions of these side chains in relation to the interfacial beta strands and the monomer as a whole.
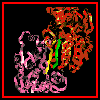 The symmetry of the monomer-
monomer interaction is shown here.
The symmetry of the monomer-
monomer interaction is shown here.
A larger scale diagram of the dimer is shown.
Consider how precise the interaction surface of the monomer must be in order for this dimerization to occur.
Note that in other organisms the enzyme exists in different multimeric forms. For example, the subunit of yeast alcohol dehydrogenase has a high degree of homology with the horse liver protein, but is tetrameric.
Back to Main PPS Index
J. Walshaw