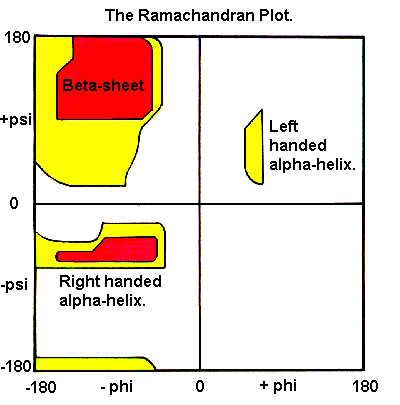
In a polypeptide the main chain N-Calpha and Calpha-C bonds relatively are free to rotate. These rotations are represented by the torsion angles phi and psi, respectively.
G N Ramachandran used computer models of small polypeptides to systematically vary phi and psi with the objective of finding stable conformations. For each conformation, the structure was examined for close contacts between atoms. Atoms were treated as hard spheres with dimensions corresponding to their van der Waals radii. Therefore, phi and psi angles which cause spheres to collide correspond to sterically disallowed conformations of the polypeptide backbone.

In the diagram above the white areas correspond to conformations where atoms in the polypeptide come closer than the sum of their van der Waals radi. These regions are sterically disallowed for all amino acids except glycine which is unique in that it lacks a side chain. The red regions correspond to conformations where there are no steric clashes, ie these are the allowed regions namely the alpha-helical and beta-sheet conformations. The yellow areas show the allowed regions if slightly shorter van der Waals radi are used in the calculation, ie the atoms are allowed to come a little closer together. This brings out an additional region which corresponds to the left-handed alpha-helix.
L-amino acids cannot form extended regions of left-handed helix but occassionally individual residues adopt this conformation. These residues are usually glycine but can also be asparagine or aspartate where the side chain forms a hydrogen bond with the main chain and therefore stabilises this otherwise unfavourable conformation. The 3(10) helix occurs close to the upper right of the alpha-helical region and is on the edge of allowed region indicating lower stability.
Disallowed regions generally involve steric hindrance between the side chain C-beta methylene group and main chain atoms. Glycine has no side chain and therefore can adopt phi and psi angles in all four quadrants of the Ramachandran plot. Hence it frequently occurs in turn regions of proteins where any other residue would be sterically hindered.
A reverse turn is region of the polypeptide having a hydrogen bond from one main chain carbonyl oxygen to the main chain N-H group 3 residues along the chain (ie O(i) to N(i+3)). Helical regions are excluded from this definition and turns between beta-strands form a special class of turn known as the beta-hairpin (see later). Reverse turns are very abundant in globular proteins and generally occur at the surface of the molecule. It has been suggested that turn regions act as nucleation centres during protein folding.
Reverse turns are divided into classes based on the phi and psi angles of the residues at positions i+1 and i+2. Types I and II shown in the figure below are the most common reverse turns, the essential difference between them being the orientation of the peptide bond between residues at (i+1) and (i+2).
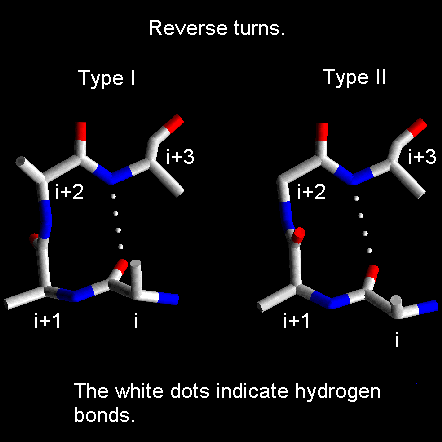
The torsion angles for the residues (i+1) and (i+2) in the two types of turn lie in distinct regions of the Ramachandran plot.
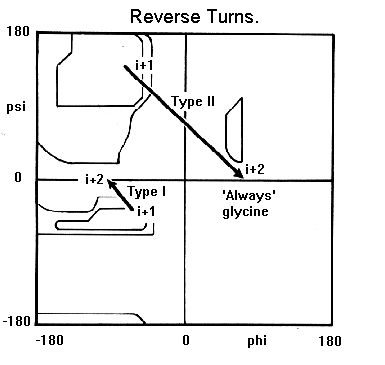
Note that the (i+2) residue of the type II turn lies in a region of the Ramachandran plot which can only be occupied by glycine. From the diagram of this turn it can be seen that were the (i+2) residue to have a side chain, there would be steric hindrance with the carbonyl oxygen of the preceding residue. Hence, the (i+2) residue of type II reverse turns is nearly always glycine.
Beta-hairpin turns occur between two antiparallel beta-strands as shown schematically below.
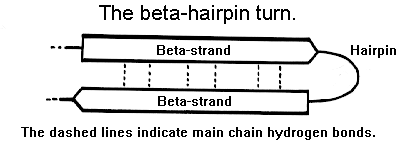
70% of these hairpin turns are shorter than seven residues in length and turns containing two residues form the largest single component. In this class, types I' and II' are the most common.
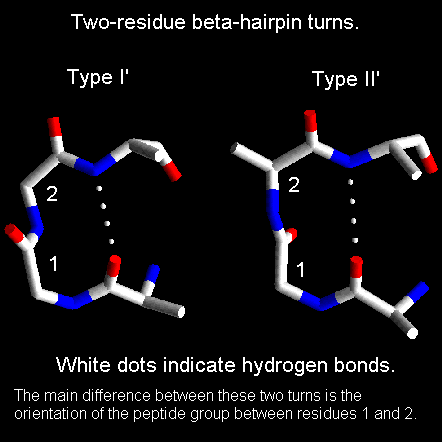
The residues forming these two-residue turns have torsion angles in characteristic regions of the Ramachandran plot.
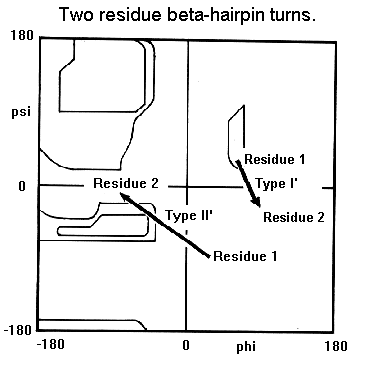
For type I' turns, residue 2 is always glycine whereas for type II' turns residue 1 is always Gly. This is because amino acids other than glycine would cause steric hindrance involving the residue's side chain and the main chain.
 Back to the Top
Back to the Top