Last modified 6th April '95 © Birkbeck College 1995
Back to main PPS Index
Back to Tertiary Structure Index
Fibrous and Structural Proteins
Collagen
In most tissues, an organised meshwork exists outside the cells: the
extracellular matrix. This protein and polysaccharide matrix acts as a
universal biological glue, as well as forming specialized structures including
tendons, bone, cartilage and basal laminae.
The matrix consists of fibrous proteins in a hydrated polysaccharide gel. The
major class of extracellular proteins consists of the collagens; in fact
collagens constitute a quarter of a mammal's entire protein content.
A collagen molecule consists of three polypeptide chains arranged in a parallel
triple-helix. The individual polypeptide chains are called alpha-chains, and
although each is helical they only have this conformation when associated
with two other alpha chains; they should not be confused with alpha-helices.
There are at least 9 distinct alpha chains; most are approximately 1000 amino
acids in length. This results in a diameter of 14 Å and a length of about
300nm for a single collagen molecule.
The helical conformation of each chain is dependent on the fact that every thirdresidue is a Gly, and that the sequence is rich in Pro. A larger side chain
than Gly would prevent the close contact of the three chains. About half of the
Pro side chains are hydroxylated; the resulting residue Hydroxproline is
referred to as "Hyp". The structure is stabilised by hydrogen bonds
between the backbone amide of a Gly residue and the backbone carbonyl of
residue X, where the sequence is represented by a repeat of -Gly-X-Y-.
Collagen I, which constitutes 90% of the collagen in a mammal, consists of a
heterotrimer where 2 of the alpha-chains are identical.
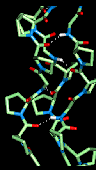 Here is a model
collagen structure.
This consists of the
repeated sequence Gly-Pro-Pro in each of the 3 alpha-chains, subjected to an
energy minimization procedure. Hydrogen bonds between the backbone atoms of the
3 alpha-chains are indicated. This model can be seen with RasMol by clicking
here .
Here is a model
collagen structure.
This consists of the
repeated sequence Gly-Pro-Pro in each of the 3 alpha-chains, subjected to an
energy minimization procedure. Hydrogen bonds between the backbone atoms of the
3 alpha-chains are indicated. This model can be seen with RasMol by clicking
here .
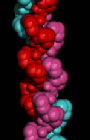 Here is a
space-filling model of the triple-helix.
Here is a
space-filling model of the triple-helix.
The alpha-chains are initially synthesized in the form of precursors called
pro-alpha-chains, which have globular sequences called extension peptides
at each end. The C-terminal extension peptides (approximately 300 residues
long) of three chains associate,
forming disulphide cross-links, and the triple helix forms in a zipper-fashion
giving procollagen. Collagen results from the cleavage of the extension peptide
domains.
Collagen type I forms collagen fibrils. Collagen molecules are arranged
head-to-tail, with a 35nm gap between each, in a staggered bundle as shown. All
the molecules point in the same direction. The -OH groups of hydroxyproline are
involved in hydrogen bonds between chains, while interactions between other
side chains are thought to be important in formation of fibrils from a number
of individual molecules. Charged and uncharged residues are found to be
periodically clustered along the sequence of collagen I every 234 residues,
which is equivalent to 67 nm; while the gap between staggered molecules in a
fibril is the same distance. This suggests that the collagen molecules are
aligned such that the maximum electrostatic and hydrophobic interactions occur
between different molecules.
The diagram here indicates
interactions between alpha-chains in two different
molecules. Again this is taken from a model structure, of the Smith collagen
microfibril arrangement in which 5 triple helices are involved in a left-handed
superhelical arrangement which is postulated to be repeated throughout the
fibril. Only two of the five collagen molecules are shown here. All the
alpha-chains consist of a repeated Gly-Pro-Hyp sequence. Three Hyp residues
are highlighted forming hydrogen bonds between individual collagen
molecules, rather than within them.
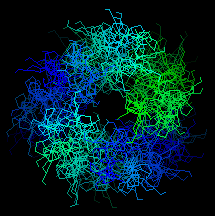 Here is an end-on view of the superheilx. Click here to see the whole
structure.
Here is an end-on view of the superheilx. Click here to see the whole
structure.
Silk fibroin, tropomyosin and keratin are other fibrous
proteins which are rich in glycine. Details of these can be found under the
Glycine Group's section on
Glycine in Structure.
Actin
At least six different types of actin are synthesized by vertebrate cells. The
different genes which code them are highly conserved however, and they have
similar properties.
Globular, or G actin is stabilized by one tightly bound calcium ion. The
protein is also associated with one molecule of ATP. The G actin polymerizes to
form filamentous or F actin; this involves hydrolysis of the terminal
phosphate of the ATP. Actin filaments consist of two strands of polymerized
actin monomers, twisted into a helix with 13.5 molecules per turn.
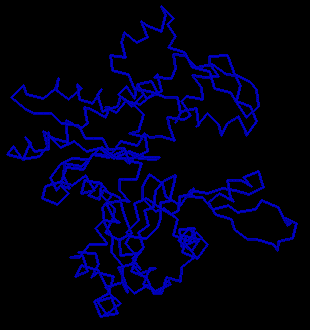 Examine the crystal structure of actin. The actin molecule is complexed
with deoxyribonuclease I; actin is the larger of the two (select Colour:chains).
Consider how the shape of this molecule might relate to its ability to
polymerize.
Examine the crystal structure of actin. The actin molecule is complexed
with deoxyribonuclease I; actin is the larger of the two (select Colour:chains).
Consider how the shape of this molecule might relate to its ability to
polymerize.
Back to the Top
Back to Main PPS Index
J. Walshaw
 Here is a model
collagen structure.
This consists of the
repeated sequence Gly-Pro-Pro in each of the 3 alpha-chains, subjected to an
energy minimization procedure. Hydrogen bonds between the backbone atoms of the
3 alpha-chains are indicated. This model can be seen with RasMol by clicking
here .
Here is a model
collagen structure.
This consists of the
repeated sequence Gly-Pro-Pro in each of the 3 alpha-chains, subjected to an
energy minimization procedure. Hydrogen bonds between the backbone atoms of the
3 alpha-chains are indicated. This model can be seen with RasMol by clicking
here . Here is a
Here is a
 Here is an end-on view of the superheilx. Click
Here is an end-on view of the superheilx. Click  Examine the crystal structure of actin.
Examine the crystal structure of actin.