Back to Protein Interactions Index
The binding of oxygen by haemoglobin is cooperative ; the protein cannot be considered in terms of four independently oxygen-binding subunits. As haemoglobin binds successive oxygens, the oxygen affinity of the subunits increases. The affinity for the fourth oxygen to bind is approximately 300 times that for the first. This cannot be explained by four independent subunits with intrinsically different affinities, as if this were the case, then oxygen would bind to the subunit with the highest intrinsic affinity; the affinity of the haemoglobin molecule would subsequently decrease rather than increase.
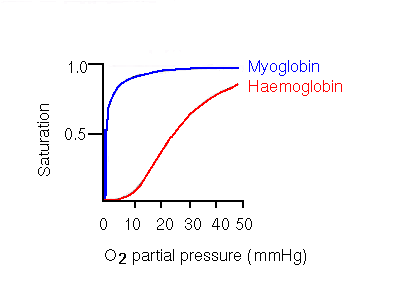
This behaviour results in an oxygen dissociation curve (a plot of saturation of oxygen-binding sites vs partial pressure of oxygen) that is sigmoidal, rather than hyperbolic. Hyperbolic curves are exhibited by monomeric molecules such as myoglobin, the monomeric alpha chain, and other non-cooperative molecules such as haemoglobin H (see below).

The significance of the sigmoidal curve is that it means that haemoglobin A becomes highly saturated at high oxygen partial pressures, and releases a significant amount of oxygen at pressures which are fairly low, but not extremely so. Contrast this to the hyperbolic myoglobin curve, where even at the low partial pressure of 20 mmHg, the oxygen-carrier is still almost totally saturated. 20mmHg is in fact the partial oxygen pressure in the capillaries of active muscles. Aterial pressure is 100mmHg. Haemoglobin A is 50% saturated at 26mmHg, while 50% saturation of myoglobin occurs at only 1mmHg.

Therefore, a cooperative binding mechanism is more efficient at collecting oxygen where it is in high concentration, and supplying it where it is needed. Tissues need be only relatively mildly deficient in oxygen for haemoglobin to give up a much more significant amount compared to a non-cooperative system such as myoglobin.
Haemoglobin may be considered to exist in two states: the tense (T) state, and the relaxed (R) state. The T form corresponds to the quaternary structure of deoxyhaemoglobin, and R to that of oxyhaemoglobin. The binding of a molecule of oxygen to a haemoglobin subunit stabilizes the R state, and thereby increases the affinity of remaining deoxygenated subunits for oxygen.
The cooperative oxygen-binding properties of haemoglobin rely on specific quaternary interactions between the subunits. In isolation, the beta subunits, form a homo-tetramer, (called haemoglobin H), which lacks the particular subunit interactions of haemoglobin A. Haemoglobin H therefore does not exhibit any cooperativity in its oxygen binding.
The allosteric mechanisms of haemoglobin A enables the affinity for oxygen to be adjusted by interactions with a number of reagents.
The oxygen affinity of haemoglobin is modified by several factors:
The various mechanisms result in haemoglobin having a lower affinity for oxygen than myoglobin.
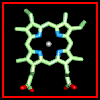 Click here for a diagram of haem.
Click here for a diagram of haem.
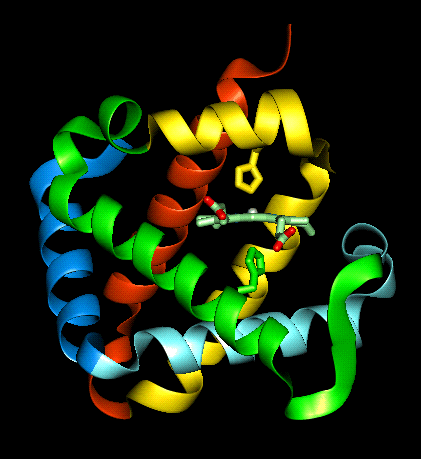
The haem group is situated between helices E and F, and is surrounded by non-polar residues except for the two carboxylate groups exposed at the protein surface (here is a diagram, and for two histidines. One of these (His 87 in alpha subunit, His 92 in beta), part of helix F, binds directly to the iron atom of the haem group (the NE2 atom of the His side chain occupies one of the six coordination positions of the iron). This His is in each subunit called the proximal histidine. The distal histidine occurs in helix E (His 58 in alpha subunit, His 63 in beta). This is near to the opposite coordination position, but does not occupy it; this coordination site is occupied by oxygen in oxyhaemoglobin.
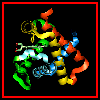 Here are orthogonal views of
(oxy)haemoglobin illustrating the positions of the proximal and distal
histidines in relation to the haem.
Here are orthogonal views of
(oxy)haemoglobin illustrating the positions of the proximal and distal
histidines in relation to the haem.
 The orientation of the four
subunits in the tetrameric haemoglobin molecule is indicated in this diagram.
The orientation of the four
subunits in the tetrameric haemoglobin molecule is indicated in this diagram.
Swiss-3D prot provide this diagram.
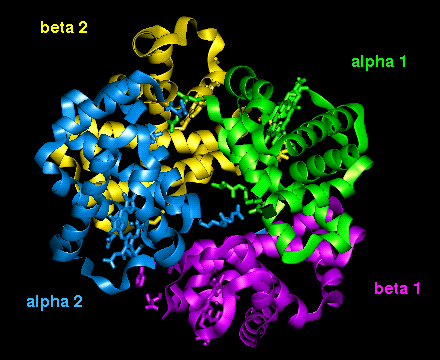 This diagram illustrates (1) and
(2) of the above.
This diagram illustrates (1) and
(2) of the above.
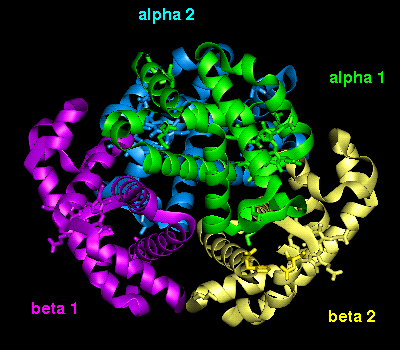 This diagram illustrates (3) and
(4).
This diagram illustrates (3) and
(4).
These interactions may also be examined in this structure which includes side chains only for the residues mentioned above .
Here is the complete structure of deoxyhaemoglobin.
The haem group of deoxyhaemoglobin is domed rather than planar. This relates to the ionic radius of the iron, which is in a high-spin Fe(II) state. The iron is too large (radius 2.06Å) to fit in the ring of nitrogens with which it coordinates; it is 0.6Å out of the plane of the ring, which is therefore distorted.
 Close-up
Close-up
 and a
perpendicular view
and a
perpendicular view
In contrast to deoxyhaemoglobin, the haem group is planar and the iron ion lies in the plane of the ring, as it is in a low-spin Fe(II) state with a smaller radius (1.98Å). All six coordination positions of the ion are occupied: the bound oxygen molecule accounts for the sixth.
Examine the structure of oxyhaemoglobin (tetramer provided by Brookhaven).
Perpendicular view:
The important interfacial structural changes are those between the two heterodimers (i.e. between the alpha 1 - beta 1 dimer, and the alpha 2 - beta 2 dimer), rather than between the two subunits within each of these dimers. Note that three of the four salt bridges listed in the section on the T state occur between the two heterodimers. Different hydrogens bonds stabilize the alpha 1 - beta 2 interface in the two different states (wot are they?). There is a 15° rotation of the two heterodimers relative to each other, as indicated below.
 Click here for diagram.
Click here for diagram.
Therefore, binding of an oxygen molecule to the Fe ion in the haem ring leads to marked structural changes elsewhere in the molecule. The R-state tertiary structure of an oxygenated subunit stabilizes the same tertiary structure in the other chains, which therefore have a higher affinity for oxygen.
Because the key to cooperativity is the interaction between heterodimers, mutations in the interfacial residues between subunits alpha 1 and beta 1, and between alpha 2 and beta 2, do not tend to affect the cooperative binding system in the same way that mutations in the alpha 1 - beta 2 interface do.
The significance of the contacts between heterodimers also explains the observed increase in oxygen affinity. Suppose that a single subunit, say alpha 1, binds an oxygen and alters from the T to the R state. Bearing in mind the list of broken salt bridges listed previously, the interactions between (a) alpha 1 and alpha 2, and (b) alpha 1 and beta 2, will change. This would result in the R state tertiary structure in subunits alpha 2 and beta 2, so that the affinity of both for oxygen is increased. Indeed, the affinity for the third oxygen molecule is not markedly higher than that for the second, but both are much greater than that for the first. With oxygens now bound to alpha 1, alpha 2 and beta 2, the R state tertiary structure will also be stabilized in beta 1 (due to the tertiary structure of alpha 2 breaking the salt bridges between it and beta 1; refer to the previous list). The final empty chain will thus have a very stable R tertiary structure; this results in the dramatically higher affinity for the fourth oxygen.
Back to Main PPS Index
J. Walshaw