Part V. A Closer Look at Glycogen Phosphorylase
The activation of the glycogenolysis pathway via the
cascade of phosphorylation reactions was illustrated in the
preceeding pages. Glycogen phosphorylase
is the ultimate enzyme in the cascade which catalyzes the
degradation of glycogen to glucose-1-phosphate.
Glycogen phosphorylase is also called phosphorylase and
in it's functionally active form is composed of a dimer of two identical
monomers each with molecular weight of 97,444 daltons.
Phosphorylation of phosphorylase
occurs on serine-14 and converts the inactive phosphorylase b
to the active phosphorylase a.
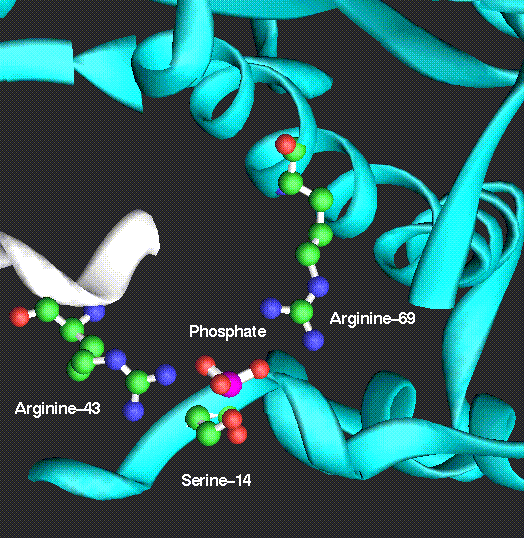
Not only is phosphorylase
regulated by phosphorylation, it is allosterically regulated
by small molecule activators and inhibitors.
Crystallographic structural studies on rabbit muscle phosphorylase
have yielded a wealth of information contributing to the
understanding of the mechanism of catalytic activation due to phosphorylation.
The structural studies were done in the laboratories of Louise Johnson
and co-workers and Robert Fletterick and co-workers.
BACK
NEXT
TABLE OF CONTENTS

