Part I. Introduction
Proteins undergo a huge number of post translational
modifications. However, only a subset of these modifications
are reversible, covalent modifications
such as acetylation, fatty acid
acylation, glycosylation and phosphorylation.
These modifications
affect the activity, life span,
or cellular location of the modified proteins.
Of the post translational modifications mentioned above,
phosphorylation is an important and ubiquitous one.
Approximately 10% of the proteins in the cell cytosol
is phosphorylated. The phosphorylation reaction in the
cell is a reversible one where kinases catalyze the
addition of the phosphoryl group and phosphatases
catalyze the removal of the phosphoryl group.
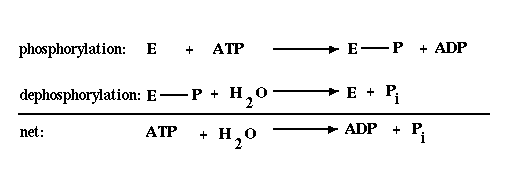
The reaction involves ATP as the phosphoryl
donor in the phosphorylation reaction and hydrolysis of the
phosphoryl group in the dephosphorylation reaction. The net result
of these reactions can be viewed as the hydrolysis of ATP, which
has a 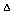 G of -12 kcal/mol under cellular conditions
and is therefore, energetically favorable.
G of -12 kcal/mol under cellular conditions
and is therefore, energetically favorable.
Residues which are the usual
sites of phosphorylation are serine, threonine and tryosine hydroxyl groups.
Aspartic acid, histidine and lysine can also be phosphorylated.
BACK
NEXT
TABLE OF CONTENTS

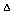 G of -12 kcal/mol under cellular conditions
and is therefore, energetically favorable.
G of -12 kcal/mol under cellular conditions
and is therefore, energetically favorable.
