My apologies to all the protein chemist to use an inorganic structure as an example. But as my goal is to give a broader view to the term hydrogen bond, this should be excused.
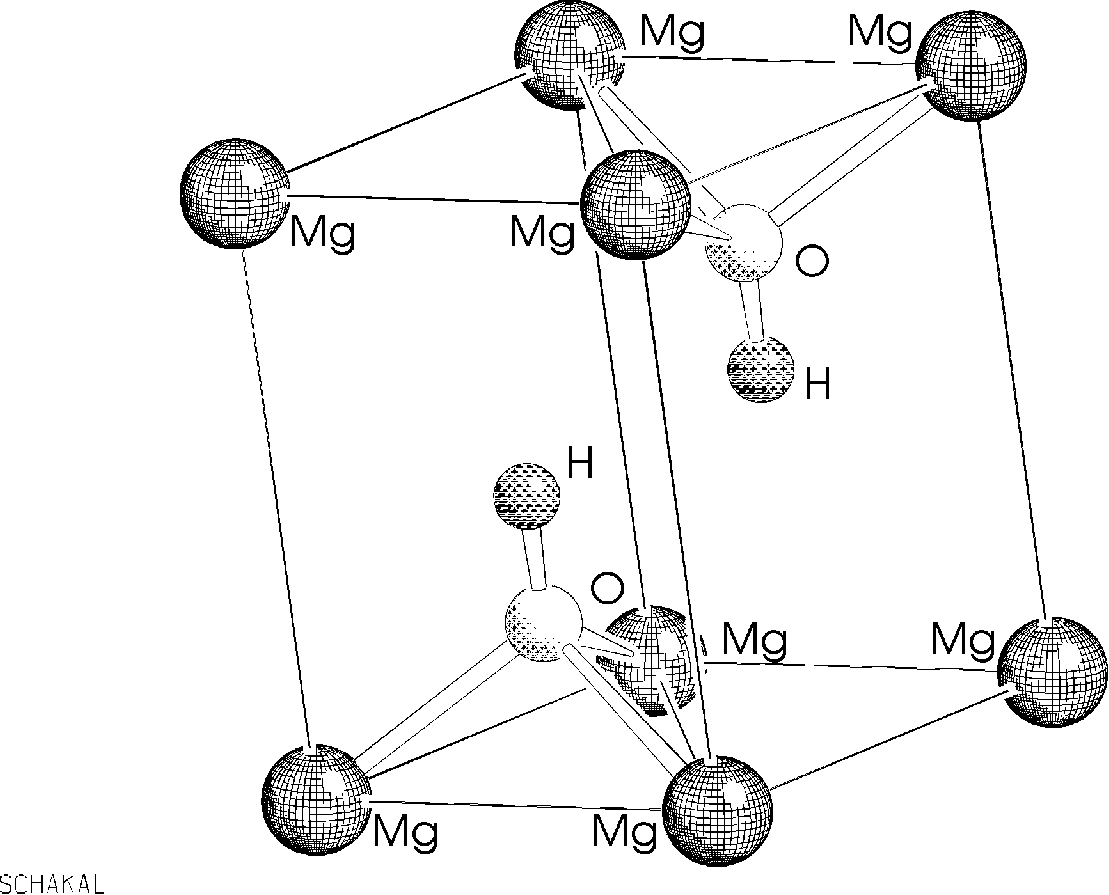
In addition the brucite structure shows some properties which are not commonly found in organic structures, which are mainly a high symmetry and a small unit cell with not many electrons in it.
Brucite is a mineral and the unit cell is shown below:

The structure is made of Mg layers coordinated octahedral by O-H groups with the hydrogen pointing in the direction of the next layer. A better view of this is given by the mirror-plane diagonal through the unit cell.
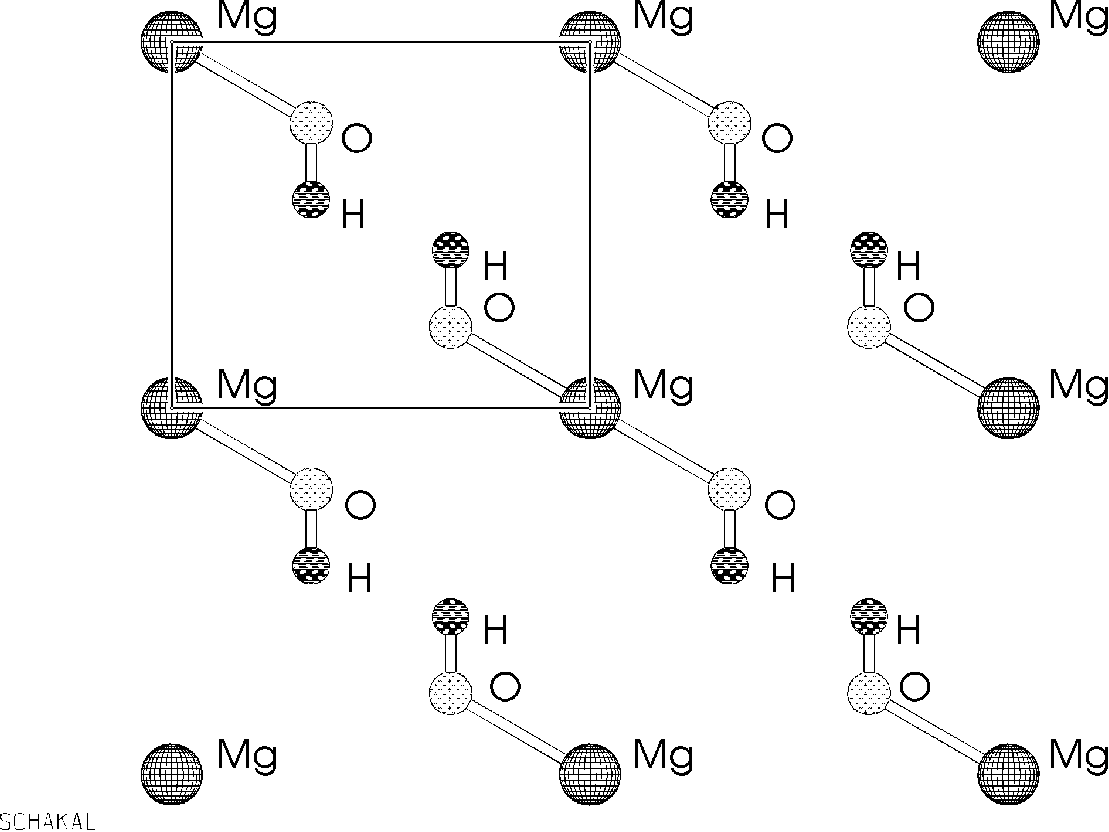
Weak hydrogen bonds between each layers seem the only possible explanation that the structure is stable, even when this 'hydrogen bonds' lie outside the normal accepted areas (see example 3 in chapter 2).
Brucite has been investigated by all three models described in chapter 3.

| 
| 
|
Last Updated: 26 October 1996