 Sheet-Sheet Packing
Sheet-Sheet Packing
![]() Index to Course Material
Index to Course Material
![]() Index to Section 9
Index to Section 9
![]() Helix-Helix Packing
Helix-Helix Packing
![]() Beta-strand Packing in Alpha/Beta Barrels
Beta-strand Packing in Alpha/Beta Barrels
Residues are approximately 7.0Å apart along strands, but approximately 4.5Å apart between adjacent strands. This means that it is not possible to produce a regular pattern of intercalation, except in almost perfectly aligned sheets (see below). In all other cases, medium sized residues form the best interface, as very large or small residues make irregular contacts which are only occasional. Of course, there is a tendency for residues buried at the interface to have hydrophobic side chains; thus the residues are often valine, leucine and isoleucine. There are nevertheless numerous exceptions, for example polar side chain atoms may be observed pointing out of the end of the sandwich. However, the general tendency is for the two packing surfaces to be flat and hydrophobic.
Pairs of sheets which pack together may be approximately aligned, or orthogonal.
In aligned beta 'sandwiches', the mean angle between the 2 sheets is in fact approximately 30° (designated -30° because the uppermost sheet is rotated clockwise with respect to the lower). The two sheets are usually independent in that the linking residues between them are not in beta sheet conformation. The angle between the sheets is determined by their right-handed twist. The observed angle varies between -20° and -50°; this is due to variation in the twist. Also side-chains are not always ideally aligned at the interface.
The sidechains in each strand alternatively face towards and away from the interface with the sheet packing against it. Whereas in untwisted sheets, side chains would pack primarily against those in the strand directly opposite, the twist means that sidechains pack against residues opposite their neighbouring strands as well. This is illustrated in Figure 1.
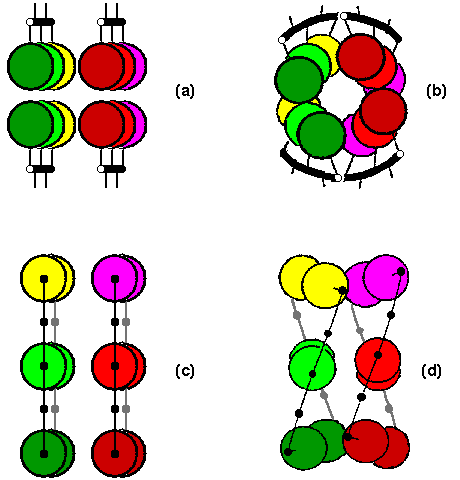
Figure 1. (a) and (c) respectively show views from the end, and from above, two untwisted two-stranded beta sheets. However in proteins the angle between two such sheets is usually approximately 30°, due to the twist, shown from the end in (b) and from above in (d). After Chothia (1984)
![]() Download the structure of a fragment of
an IgM antibody (Fan et al., 1992):
1igm (172Kb)
[Bbk|BNL|ExP|Waw|Hal]
Note that there are two beta-sandwich domains in the asymmetric unit, termed the
L (light) and H (heavy) chains. We will look at the L chain.
Download the structure of a fragment of
an IgM antibody (Fan et al., 1992):
1igm (172Kb)
[Bbk|BNL|ExP|Waw|Hal]
Note that there are two beta-sandwich domains in the asymmetric unit, termed the
L (light) and H (heavy) chains. We will look at the L chain.
Click below for the RasMol scripts which colour the two sheets in this domain, and display the side chains of the packing interface in each:
In each case, only the side chains are displayed as space-filling atoms. You may also download these images of the resulting views:Note that although the contact surface is largely hydrophobic, both sheets have at least one polar side chain in the interface. To display these, use the command select sidechain and (nitrogen, oxygen) command followed by colour white to highlight these. There is a disulphide bond between Cys 23 (sheet 1) and Cys 88 (sheet 2); try select sulphur followed by colour yellow to display the atoms involved.
After you have run script 2 above, copy and paste
the following commands to show both sheets together; or download this
image (12Kb).
The following diagrams indicate how these sheets fit together:
large (55Kb) or small (18Kb).
NOTE: the arrows are not intended to indicate a folding pathway, merely
the orientation of the sheets.
If you want to render the main chain of the strands as spacefilling as well,
use these commands:
You can then see the 4 layers of atoms (backbone,sidechain,sidechain,backbone)
as in this diagram.
By selecting a suitable view, can you determine with which polar groups the polar side chain atoms of Gln 6, Gln 37, Arg 47 and Tyr 86 interact?
There is also a script to colour code the direction of each strand: yellow and grey (sheet 1) and red and green (sheet 2).
Orthogonal beta sheet packings consist of beta sheets folded on themselves; the two sheets make an angle of -90°. The strands at one corner or 2 diagonally opposite corners pass uninterrupted from one layer to the other. Local coiling at the corner or a beta bulge facilitates the right-angled bend. These bends are right-handed, due to permitted phi and psi angles. Figure 2 below illustrates this model.
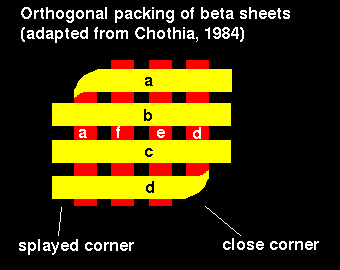
Figure 2. Orthogonal beta sheet packing. Only along one diagonal do the two sheets make contact. Large side-chains in loops usually fill the spaces between the splayed corners. After Chothia, 1984
The script highlights the packing interface with the side chains rendered as space filling atoms, and the main chain as ribbons. The sandwich is shown from the side. The following images show the interface from the side (11Kb) and end (10Kb).
Make sure you can identify the diagonal joining the two 'close corners'. Are all of the sidechains at the interface completely hydrophobic? If not, with what other polar groups do the polar atoms appear to interact?
Copy and paste the following commands to
render the backbone atoms as space-filling as well, to illustrate the 4-layered
structure:
There are also these images of side (16Kb) and
end (16Kb) views.
Last updated 1st August '96