 Domain Folds
Domain Folds
![]() Index to Course Material
Index to Course Material
![]() Index to Section 10
Index to Section 10
![]() All Beta Folds
All Beta Folds
In addition, it is worthwhile browsing through the Mainly Alpha Class of the CATH Protein Structure Classification Database" at University College, London. This lists 9 orthogonal, and 3 aligned topologies of alpha fold, as well as a solenoid architecture.
You should also be aware of alternative classifications, such as the Structural Classification of Proteins database (Alexey G. Murzin, Steven E. Brenner, Tim J.P. Hubbard, and Cyrus Chothia). In all, seventy-one categories of all alpha folds are listed. A number of the entries have links to diagrams by Manuel Peitsch.
With MAGE installed, study this Kinemage on Alpha Domain Structures, which accompanies the Branden and Tooze book.
![]() Download the glucagon structure:
1gcn (25Kb)
[Bbk|BNL|ExP|Waw|Hal]
Download the glucagon structure:
1gcn (25Kb)
[Bbk|BNL|ExP|Waw|Hal]
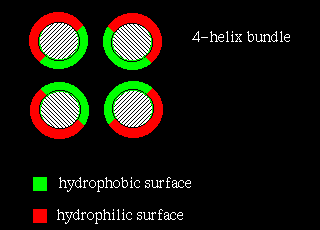
In four-helix-bundle proteins the interfaces between the helices consist mostly of hydrophobic residues while polar side chains on the exposed surfaces interact with the aqueous environment, as indicated below:
![]() After downloading this ferritin structure, leave the RasMol window open
as we will examine it again shortly.
1fha (121Kb)
[Bbk|BNL|ExP|Waw|Hal]
This SCRIPT colours the side chains of the main part
of the 4 helices (the 4 residues at each end are not shown) as in the diagram
above. 16Kb GIF image.
After downloading this ferritin structure, leave the RasMol window open
as we will examine it again shortly.
1fha (121Kb)
[Bbk|BNL|ExP|Waw|Hal]
This SCRIPT colours the side chains of the main part
of the 4 helices (the 4 residues at each end are not shown) as in the diagram
above. 16Kb GIF image.
Compare this with the arrangement of residues that would be expected in a membrane-spanning helical domain, which is indicated elsewhere in Section 10 (page on membrane proteins) . The central helices of the photosynthetic reaction centre in fact have a similar arrangement to the four- helix bundle.
Other examples exhibit a much more open packing arrangement, as in the steroid-binding
proteins uteroglobin, and ![]() Clara cell 17kDa protein : 1ccd (56Kb)
[Bbk|BNL|ExP|Waw|Hal]
Clara cell 17kDa protein : 1ccd (56Kb)
[Bbk|BNL|ExP|Waw|Hal]
14.6Kb GIF.
 Click here for a
diagram of
myohemerythrin, which has this fold.
Click here for a
diagram of
myohemerythrin, which has this fold.
![]() To obtain a similar rendition as in the diagram, use the colour group
and ribbon commands: 2mhr (201Kb)
[Bbk|BNL|ExP|Waw|Hal]
To obtain a similar rendition as in the diagram, use the colour group
and ribbon commands: 2mhr (201Kb)
[Bbk|BNL|ExP|Waw|Hal]
(other examples are cytochrome c': 2ccy (194Kb)
[Bbk|BNL|ExP|Waw|Hal]
and cytochrome b-562: 256b (174Kb)
[Bbk|BNL|ExP|Waw|Hal]
- which have two molecules in the asymmetric unit; use restrict *a to
show only the A chain).
A more complex arrangement is possible:
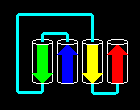 Click here for the
four-helix bundle topology of ferritin.
Click here for the
four-helix bundle topology of ferritin.
![]() If you still have the RasMol window
with the ferritin structure (see above), type:
If you still have the RasMol window
with the ferritin structure (see above), type:
select wireframe off ribbon colour groupOtherwise,
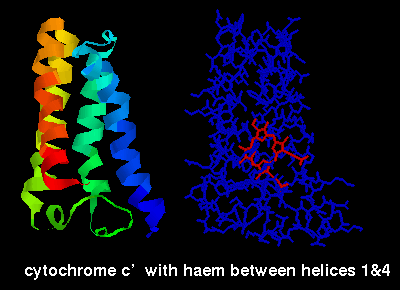
The ligand-binding sites of these proteins occur between the alpha helices.
The ligands can be seen by selecting the structures listed above and choosing
the restrict *a, wireframe and colour chain options, e.g. below is a diagram of
cytochrome C' (2ccy- see above for link) indicating the haem group,
which is sited between the first and fourth helices.
Transcription factors are proteins which bind to control regions of DNA. These regions are "upstream" of the structural gene (the sequence which actually codes for a protein) whose transcription they regulate. Transcription factors have a DNA-binding domain and a domain that activates transcription.
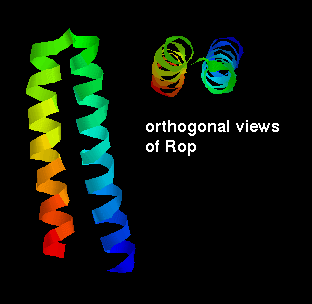
The RNA-binding two-helix protein Rop has already been mentioned. A three-helix
bundle forms the basis of a DNA-binding domain which occurs in a number of
proteins- for example homeodomain proteins.
![]() Examine the crystal structure
of engrailed homeodomain binding to DNA,
1hdd (154Kb)
[Bbk|BNL|ExP|Waw|Hal]
and
three
different
diagrams courtesy of Manuel Peitsch.
Examine the crystal structure
of engrailed homeodomain binding to DNA,
1hdd (154Kb)
[Bbk|BNL|ExP|Waw|Hal]
and
three
different
diagrams courtesy of Manuel Peitsch.
![]() Click here for the structure of the cro repressor from phage 434.
2cro (49Kb)
[Bbk|BNL|ExP|Waw|Hal]
Click here for the structure of the cro repressor from phage 434.
2cro (49Kb)
[Bbk|BNL|ExP|Waw|Hal]
Also refer to the GCN4 transcription factor leucine zipper described in the section on coiled coils, by Antti Iivanainen of the Department of Medical Biochemistry and Biophysics, Karolinska Institute, Stockholm.
![]()
Last updated 11th Jun '96